Summary
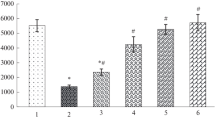
The mechanisms of glycolytic rate control during hibernation in the ground squirrel Spermophilus lateralis were investigated in four tissues: heart, liver, kidney, and leg muscle. Overall glycogen phosphorylase activity decreased significantly in liver and kidney to give 50% or 75% of the activity found in the corresponding euthermic organs, respectively. The concentration of fructose-2,6-bisphosphate (F-2,6-P2) decreased significantly in heart and leg muscle during hibernation to 50% and 80% of euthermic tissue concentrations, respectively, but remained constant in liver and kidney. The overall activity of pyruvate dehydrogenase (PDH) in heart and kidney from hibernators was only 4% of the corresponding euthermic values. Measurements of phosphofructokinase (PFK) and pyruvate kinase (PK) kinetic parameters in euthermic and hibernating animals showed that heart and skeletal muscle had typical rabbit skeletal M-type PFK and M1-type PK. Liver and kidney PFK were similar to the L-type enzyme from rabbit liver, whereas liver and kidney PK were similar to the M2 isozyme found primarily in rabbit kidney. The kinetic parameters of PFK and PK from euthermic vs hibernating animals were not statistically different. These data indicate that tissue-specific phosphorylation of glycogen phosphorylase and PDH, as well as changes in the concentration of F-2,6-P2 may be part of a general mechanism to coordinate glycolytic rate reduction in hibernating S. lateralis.
Similar content being viewed by others
Abbreviations
- ADP:
-
adenosine diphosphate
- AMP:
-
adenosine monophosphate
- ATP:
-
adenonine triphoshate
- EDTA:
-
ethylenediaminetetra-acetic acid
- EGTA:
-
ethylene glycol tetra-acetic acid
- F-6-P:
-
fructose 6-phosphate
- F-1,6-P2 :
-
fructose 1,6-bisphosphate
- F-2,6-P2 :
-
fructose-2,6-bisphosphate
- K a :
-
activation coefficient
- I50 :
-
concentration of inhibitor which reduces control activity by 50%
- PDH:
-
pyruvate dehydrogenase
- PEP:
-
phosphoenolpyruvate
- PFK:
-
6-phosphofructo-1-kinase
- PK:
-
pyruvate kinase
References
Behrish HW (1974) Temperature and the regulation of enzyme activity in the hibernator. Isoenzymes of liver pyruvate kinase from the hibernating and non-hibernating arctic ground squirrel. Can J Biochem 52:894–902
Borgmann AI, Moon TW (1976) Enzymes of the normothermic and hibernating bat Myotis lucifugus: temperature as a modulator of pyruvate kinase. J Comp Physiol 107:185–200
Deavers DR, Musacchia XJ (1980) Water metabolism and renal function during hibernation and hypothermia. Fed Proc Fed Am Soc Exp Biol 39:2969–2973
Denton RD, McCormack JG, Midgley PJW, Rutter GA (1987) Hormonal regulation of fluxes through pyruvate dehydrogenase and the citric acid cycle in mammalian tissues. Biochem Soc Symp 54:127–143
Dunaway GA (1983) A review of animal phosphofructokinase isozymes with an emphasis on their physiological role. Mol Cell Biochem 52:75–91
Elnageh KM, Gaitonde MK (1988) Effect of a deficiency of thiamine on brain pyruvate dehydrogenase: enzyme assay by three different methods. J Neurochem 51:1482–1489
Engstrom L, Ekman P, Humble E, Zetterqvist O (1987) Pyruvate kinase. In: Boyer P (ed) The enzymes, vol XVIII. Academic Press, New York, pp 47–45
Friedrich P (1988) Supramolecular enzyme organization. Pergamon Press, Oxford, pp 93–178
Geiser F (1988) Reduction of metabolism during hibernation and daily torpor in mammals and birds: temperature effect or physiological inhibition: J Comp Physiol B 158:25–37
Hall ER, Cottam GL (1979) Isozymes of pyruvate kinase in vertebrates: their physical, chemical, kinetic, and immunological properties. Int J Biochem 9:785–793
Helmerhorst E, Stokes GB (1980) Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem 104:130–135
Hochachka PW, Somero GN (1984) Biochemical adaptation. Princeton University Press, Princeton, NJ
Hochachka PW, Guppy M (1987) Metabolic arrest and the control of biological time. Harvard University Press, Cambridge, Mass
Hue L (1982) Role of fructose 2,6-bisphosphate in the stimulation of glycolysis by anoxia in isolated hepatocytes. Biochem J 206:359–365
Hue L (1983) Role of fructose 2,6-bisphosphate in the regulation of glycolysis. Biochem Soc Trans 11:246–247
Lyman CP, Willis JS, Malan A, Wang LCH (1982) Hibernation and torpor in mammals and birds. Academic Press, New York
Reed LJ, Yeaman SJ (1987) Pyruvate dehydrogenase. In: Boyer PD, Krebs EG (eds) The enzymes, vol XVIII. Academic Press, New York, pp 77–95
van Schaftingen E (1984) d-Fructose 2,6-bisphosphate. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 6. Verlag Chemie, Weinheim, pp 335–341
Seubert W, Schoner W (1971) The regulation of pyruvate kinase. Curr Top Cell Regul 3:237–267
Snapp BD, Heller HC (1981) Suppression of metabolism during hibernation in ground squirrels (Citellus lateralis). Physiol Zool 54:297–307
Srivastava DK, Bernhard SA (1986) Enzyme-enzyme interactions and the regulation of metabolic reaction pathways. Curr Top Cell Regul 28:1–68
Storey KB (1987a) Regulation of liver metabolism by enzyme phosphorylation during mammalian hibernation. J Biol Chem 262:1670–1673
Storey KB (1987b) Investigations of the mechanisms of glycolytic control during hibernation. Can J Zool 65:3079–3083
Storey KB (1988a) Suspended animation: the molecular basis of metabolic depression. Can J Zool 66:124–132
Storey KB (1988b) Mechanisms of glycolytic control during facultative anaerobiosis in a marine mollusc: tissue-specific analysis of glycogen phosphorylase and fructose 2,6-bisphosphate. Can J Zool 66:1767–1771
Storey KB (1989) Integrated control of metabolic rate depression via reversible phosphorylation of enzyme in hibernating mammals. In: Malan A, Canguilhem B (eds) Living in the cold II. John Libbey Eurotext, London, pp 309–319
Tashima LS, Adelstein SJ, Lyman CP (1970) Radioglucose utilization by active, hibernating, and arousing ground squirrels. Am J Physiol 218:303–309
Wang LCH (1978) Energetic and field aspects of mammalian torpor: the Richardson's ground squirrel. In: Wang LCH, Hudson JW (eds) Strategies in cold. Natural torpidity and thermogenesis. Academic Press, New York, pp 109–145
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brooks, S.P.J., Storey, K.B. Mechanisms of glycolytic control during hibernation in the ground squirrel Spermophilus lateralis . J Comp Physiol B 162, 23–28 (1992). https://doi.org/10.1007/BF00257932
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00257932