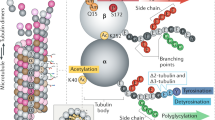
Abstract
Variability characterizes the complexity of biological systems and is essential for their function. Microtubules (MTs) play a role in structural integrity, cell motility, material transport, and force generation during mitosis, and dynamic instability exemplifies the variability in the proper function of MTs. MTs are a platform for energy transfer in cells. The dynamic instability of MTs manifests itself by the coexistence of growth and shortening, or polymerization and depolymerization. It results from a balance between attractive and repulsive forces between tubulin dimers. The paper reviews the current data on MTs and their potential roles as energy-transfer cellular structures and presents how variability can improve the function of biological systems in an individualized manner. The paper presents the option for targeting MTs to trigger dynamic improvement in cell plasticity, regulate energy transfer, and possibly control quantum effects in biological systems. The described system quantifies MT-dependent variability patterns combined with additional personalized signatures to improve organ function in a subject-tailored manner. The platform can regulate the use of MT-targeting drugs to improve the response to chronic therapies. Ongoing trials test the effects of this platform on various disorders.

Similar content being viewed by others
Data availability
All data is included and is part of public domains.
Abbreviations
- MTs:
-
Microtubules
- AFs:
-
Actin filaments
- Ifs:
-
Intermediate filaments
- GTP:
-
Guanosine-5'-triphosphate
- MAPs:
-
Microtubule-associated proteins
- PCAF:
-
P300/CBP-associated factor
- EB1:
-
End binding 1
- TIPs:
-
Plus-end tracking proteins
- IFT:
-
Intraflagellar transport
- CP110:
-
Centriolar coiled coil protein 110
- PACRG:
-
Parkin coregulated gene
- ATP:
-
Adenosine triphosphate
- ADP:
-
Adenosine diphosphate
- TTs:
-
C-terminal tails
- VDAC:
-
Voltage-dependent anion-selective channel
- Trp:
-
Tryptophan
- RET:
-
Resonance energy transfer
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system
- CaMKII:
-
Ca/calmodulin-dependent kinase II
- Orch OR:
-
Orchestrated objective reduction
- OR:
-
Objective reduction
- QMT:
-
Quantum mobility theory
- POCD:
-
Postoperative cognitive dysfunction
- CBS:
-
Colchicine binding site
- FMF:
-
Familial Mediterranean fever
References
Ilan Y (2019) Overcoming randomness does not rule out the importance of inherent randomness for functionality. J Biosci 44:1–12
Ilan Y (2020) Advanced tailored randomness: a novel approach for improving the efficacy of biological systems. J Comput Biol 27:20–29. https://doi.org/10.1089/cmb.2019.0231
Ilan Y (2019) beta-glycosphingolipids as mediators of both inflammation and immune tolerance: a manifestation of randomness in biological systems. Front Immunol 10:1143. https://doi.org/10.3389/fimmu.2019.01143
Ilan Y (2019) Generating randomness: making the most out of disordering a false order into a real one. J Transl Med 17:49. https://doi.org/10.1186/s12967-019-1798-2
Kenig A, Ilan Y (2019) A personalized signature and chronotherapy-based platform for improving the efficacy of sepsis treatment. Front Physiol 10:1542. https://doi.org/10.3389/fphys.2019.01542
Khoury T, Ilan Y (2019) introducing patterns of variability for overcoming compensatory adaptation of the immune system to immunomodulatory agents: a novel method for improving clinical response to anti-TNF therapies. Front Immunol 10:2726. https://doi.org/10.3389/fimmu.2019.02726
Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312:237–242. https://doi.org/10.1038/312237a0
Burbank KS, Mitchison TJ (2006) Microtubule dynamic instability. Curr Biol 16:R516–R517. https://doi.org/10.1016/j.cub.2006.06.044
Ilan Y (2019) Randomness in microtubule dynamics: an error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol Int 43:739–748. https://doi.org/10.1002/cbin.11157
Marais A, Adams B, Ringsmuth AK, Ferretti M, Gruber JM, Hendrikx R, Schuld M, Smith SL, Sinayskiy I, Kruger TPJ, Petruccione F, van Grondelle R (2018) The future of quantum biology. J R Soc Interface. https://doi.org/10.1098/rsif.2018.0640
Brookes JC (2017) Quantum effects in biology: golden rule in enzymes, olfaction, photosynthesis and magnetodetection. Proc Math Phys Eng Sci 473:20160822. https://doi.org/10.1098/rspa.2016.0822
Hameroff S, Penrose R (2014) Consciousness in the universe: a review of the “Orch OR” theory. Phys Life Rev 11:39–78. https://doi.org/10.1016/j.plrev.2013.08.002
Needleman DJ, Ojeda-Lopez MA, Raviv U, Ewert K, Miller HP, Wilson L, Safinya CR (2005) Radial compression of microtubules and the mechanism of action of taxol and associated proteins. Biophys J 89:3410–3423. https://doi.org/10.1529/biophysj.104.057679
Moujaber O, Stochaj U (2020) The cytoskeleton as regulator of cell signaling pathways. Trends Biochem Sci 45:96–107. https://doi.org/10.1016/j.tibs.2019.11.003
Ilan-Ber T, Ilan Y (2019) The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol Immunol 111:73–82. https://doi.org/10.1016/j.molimm.2019.04.014
Hamant O, Inoue D, Bouchez D, Dumais J, Mjolsness E (2019) Are microtubules tension sensors? Nat Commun 10:2360. https://doi.org/10.1038/s41467-019-10207-y
Ilan Y (2019) Microtubules: from understanding their dynamics to using them as potential therapeutic targets. J Cell Physiol 234:7923–7937. https://doi.org/10.1002/jcp.27978
Goodson HV, Jonasson EM (2018) Microtubules and microtubule-associated proteins. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a022608
Fischer RS, Fowler VM (2015) Thematic minireview series: the state of the cytoskeleton in 2015. J Biol Chem 290:17133–17136. https://doi.org/10.1074/jbc.R115.663716
Gildersleeve RF, Cross AR, Cullen KE, Fagen AP, Williams RC Jr (1992) Microtubules grow and shorten at intrinsically variable rates. J Biol Chem 267:7995–8006
Desai A, Mitchison TJ (1997) Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13:83–117. https://doi.org/10.1146/annurev.cellbio.13.1.83
Ayoub AT, Staelens M, Prunotto A, Deriu MA, Danani A, Klobukowski M, Tuszynski JA (2017) Explaining the microtubule energy balance: contributions due to dipole moments, charges, van der waals and solvation energy. Int J Mol Sci. https://doi.org/10.3390/ijms18102042
Sept D, Baker NA, McCammon JA (2003) The physical basis of microtubule structure and stability. Protein Sci 12:2257–2261. https://doi.org/10.1110/ps.03187503
Matov A, Applegate K, Kumar P, Thoma C, Krek W, Danuser G, Wittmann T (2010) Analysis of microtubule dynamic instability using a plus-end growth marker. Nat Methods 7:761–768. https://doi.org/10.1038/nmeth.1493
Brouhard GJ (2015) Dynamic instability 30 years later: complexities in microtubule growth and catastrophe. Mol Biol Cell 26:1207–1210. https://doi.org/10.1091/mbc.E13-10-0594
Mitchison T, Kirschner M (1984) Microtubule assembly nucleated by isolated centrosomes. Nature 312:232–237. https://doi.org/10.1038/312232a0
Mitchison TJ (2014) The engine of microtubule dynamics comes into focus. Cell 157:1008–1010. https://doi.org/10.1016/j.cell.2014.05.001
Billger MA, Bhattacharjee G, Williams RC Jr (1996) Dynamic instability of microtubules assembled from microtubule-associated protein-free tubulin: neither variability of growth and shortening rates nor “rescue” requires microtubule-associated proteins. Biochemistry 35:13656–13663. https://doi.org/10.1021/bi9616965
Gasic I, Mitchison TJ (2019) Autoregulation and repair in microtubule homeostasis. Curr Opin Cell Biol 56:80–87. https://doi.org/10.1016/j.ceb.2018.10.003
Benarroch EE (2016) Dynamics of microtubules and their associated proteins: recent insights and clinical implications. Neurology 86:1911–1920. https://doi.org/10.1212/WNL.0000000000002686
Ward T, Wang M, Liu X, Wang Z, Xia P, Chu Y, Wang X, Liu L, Jiang K, Yu H, Yan M, Wang J, Hill DL, Huang Y, Zhu T, Yao X (2013) Regulation of a dynamic interaction between two microtubule-binding proteins, EB1 and TIP150, by the mitotic p300/CBP-associated factor (PCAF) orchestrates kinetochore microtubule plasticity and chromosome stability during mitosis. J Biol Chem 288:15771–15785. https://doi.org/10.1074/jbc.M112.448886
Xia P, Wang Z, Liu X, Wu B, Wang J, Ward T, Zhang L, Ding X, Gibbons G, Shi Y, Yao X (2012) EB1 acetylation by P300/CBP-associated factor (PCAF) ensures accurate kinetochore-microtubule interactions in mitosis. Proc Natl Acad Sci USA 109:16564–16569. https://doi.org/10.1073/pnas.1202639109
Kuhlman SJ, Craig LM, Duffy JF (2018) Introduction to chronobiology. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a033613
Qian J, Gelens L, Bollen M (2019) Coordination of timers and sensors in cell signaling. BioEssays 41:e1800217. https://doi.org/10.1002/bies.201800217
Mora-Santos MD, Hervas-Aguilar A, Sewart K, Lancaster TC, Meadows JC, Millar JB (2016) Bub3-Bub1 binding to Spc7/KNL1 toggles the spindle checkpoint switch by licensing the interaction of Bub1 with Mad1-Mad2. Curr Biol 26:2642–2650. https://doi.org/10.1016/j.cub.2016.07.040
Anderson CA, Eser U, Korndorf T, Borsuk ME, Skotheim JM, Gladfelter AS (2013) Nuclear repulsion enables division autonomy in a single cytoplasm. Curr Biol 23:1999–2010. https://doi.org/10.1016/j.cub.2013.07.076
Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST (2019) Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol 15:199–219. https://doi.org/10.1038/s41581-019-0116-9
Lechtreck KF, Van De Weghe JC, Harris JA, Liu P (2017) Protein transport in growing and steady-state cilia. Traffic 18:277–286. https://doi.org/10.1111/tra.12474
Yadav SP, Sharma NK, Liu C, Dong L, Li T, Swaroop A (2016) Centrosomal protein CP110 controls maturation of the mother centriole during cilia biogenesis. Development 143:1491–1501. https://doi.org/10.1242/dev.130120
Loucks CM, Bialas NJ, Dekkers MP, Walker DS, Grundy LJ, Li C, Inglis PN, Kida K, Schafer WR, Blacque OE, Jansen G, Leroux MR (2016) PACRG, a protein linked to ciliary motility, mediates cellular signaling. Mol Biol Cell 27:2133–2144. https://doi.org/10.1091/mbc.E15-07-0490
Gasic I, Boswell SA, Mitchison TJ (2019) Tubulin mRNA stability is sensitive to change in microtubule dynamics caused by multiple physiological and toxic cues. PLoS Biol 17:e3000225. https://doi.org/10.1371/journal.pbio.3000225
Hameroff SR, Craddock TJ, Tuszynski JA (2014) Quantum effects in the understanding of consciousness. J Integr Neurosci 13:229–252. https://doi.org/10.1142/S0219635214400093
Heurs M (2018) Gravitational wave detection using laser interferometry beyond the standard quantum limit. Philos Trans A Math Phys Eng Sci. https://doi.org/10.1098/rsta.2017.0289
Torday JS (2018) Quantum mechanics predicts evolutionary biology. Prog Biophys Mol Biol 135:11–15. https://doi.org/10.1016/j.pbiomolbio.2018.01.003
Aerts D (2014) Quantum theory and human perception of the macro-world. Front Psychol 5:554. https://doi.org/10.3389/fpsyg.2014.00554
Hameroff SR (2018) Anesthetic action and “quantum consciousness”: a match made in olive oil. Anesthesiology 129:228–231. https://doi.org/10.1097/ALN.0000000000002273
Craddock TJ, Tuszynski JA (2010) A critical assessment of the information processing capabilities of neuronal microtubules using coherent excitations. J Biol Phys 36:53–70. https://doi.org/10.1007/s10867-009-9158-8
Nicosia A, Nikas DN, Castriota F, Reimers B (2009) Diagnosis and management of periprocedural complications of carotid artery stenting. G Ital Cardiol (Rome) 10:718–724
Harney M (2020) The effects of quantum entanglement on chromatin and gene expression. Adv Exp Med Biol 1195:73–76. https://doi.org/10.1007/978-3-030-32633-3_11
Wen XG (2019) Choreographed entanglement dances: Topological states of quantum matter. Science. https://doi.org/10.1126/science.aal3099
Axelrod S, Brumer P (2019) Multiple time scale open systems: reaction rates and quantum coherence in model retinal photoisomerization under incoherent excitation. J Chem Phys 151:014104. https://doi.org/10.1063/1.5099969
Majumdar S, Pal S (2017) Bacterial intelligence: imitation games, time-sharing, and long-range quantum coherence. J Cell Commun Signal 11:281–284. https://doi.org/10.1007/s12079-017-0394-6
Badra NK (2019) Revised standard model of physics and origin of biology. J Appl Phys 11:12–40
Frohlich H (1968) Long range coherence and energy storage in biological systems. Int J Quant Chem 2:641–649
Demetrius L (2003) Quantum statistics and alometric scaling of organisms. Physica A 322:477–490
Davies P, Demetrius LA, Tuszynski JA (2012) Implications of quantum metabolism and natural selection for the origin of cancer cells and tumor progression. AIP Adv 2:11101. https://doi.org/10.1063/1.3697850
Mailloux RJ (2015) Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol 4:381–398. https://doi.org/10.1016/j.redox.2015.02.001
Engel GS, Calhoun TR, Read EL, Ahn TK, Mancal T, Cheng YC, Blankenship RE, Fleming GR (2007) Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446:782–786. https://doi.org/10.1038/nature05678
Craddock TJ, Friesen D, Mane J, Hameroff S, Tuszynski JA (2014) The feasibility of coherent energy transfer in microtubules. J R Soc Interface 11:20140677. https://doi.org/10.1098/rsif.2014.0677
Zhou C, Shi Q, Guo W, Terrell L, Qureshi AT, Hayes DJ, Wu Q (2013) Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl Mater Interfaces 5:3847–3854. https://doi.org/10.1021/am4005072
Dudev T, Lim C (2010) Factors governing the Na(+) vs K(+) selectivity in sodium ion channels. J Am Chem Soc 132:2321–2332. https://doi.org/10.1021/ja909280g
MacKinnon R (2003) Potassium channels. FEBS Lett 555:62–65. https://doi.org/10.1016/s0014-5793(03)01104-9
Vaziri AP, M.B. (2010) Quantum coherence in ion channels: resonances, transport and verification. New J Phys 12:085001
Schmidt MI, Ruedenberg J, K, (2014) Covalent bonds are created by the drive of electron waves to lower their kinetic energy through expansion. J Chem Phys 28:204104
Albrecht-Buehler G (1998) Altered drug resistance of microtubules in cells exposed to infrared light pulses: are microtubules the “nerves” of cells? Cell Motil Cytoskeleton 40:183–192. https://doi.org/10.1002/(SICI)1097-0169(1998)40:2%3c183::AID-CM7%3e3.0.CO;2-G
Barvitenko N, Lawen A, Aslam M, Pantaleo A, Saldanha C, Skverchinskaya E, Regolini M, Tuszynski JA (2018) Integration of intracellular signaling: biological analogues of wires, processors and memories organized by a centrosome 3D reference system. Biosystems 173:191–206. https://doi.org/10.1016/j.biosystems.2018.08.007
Santelices IB, Friesen DE, Bell C, Hough CM, Xiao J, Kalra A, Kar P, Freedman H, Rezania V, Lewis JD, Shankar K, Tuszynski JA (2017) Response to alternating electric fields of tubulin dimers and microtubule ensembles in electrolytic solutions. Sci Rep 7:9594. https://doi.org/10.1038/s41598-017-09323-w
Friesen DE, Craddock TJ, Kalra AP, Tuszynski JA (2015) Biological wires, communication systems, and implications for disease. Biosystems 127:14–27. https://doi.org/10.1016/j.biosystems.2014.10.006
Tuszynski JA, Portet S, Dixon JM, Luxford C, Cantiello HF (2004) Ionic wave propagation along actin filaments. Biophys J 86:1890–1903. https://doi.org/10.1016/S0006-3495(04)74255-1
Poznanski RR, Cacha LA, Al-Wesabi YMS, Ali J, Bahadoran M, Yupapin PP, Yunus J (2017) Erratum: solitonic conduction of electrotonic signals in neuronal branchlets with polarized microstructure. Sci Rep 7:10675. https://doi.org/10.1038/s41598-017-07626-6
Kalra AP, Eakins BB, Patel SD, Ciniero G, Rezania V, Shankar K, Tuszynski JA (2020) All wired up: an exploration of the electrical properties of microtubules and tubulin. ACS Nano. https://doi.org/10.1021/acsnano.0c06945
Shen J, Zhang JH, Xiao H, Wu JM, He KM, Lv ZZ, Li ZJ, Xu M, Zhang YY (2018) Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis 9:81. https://doi.org/10.1038/s41419-017-0145-x
Shen C, Guo W (2018) Ion permeability of a microtubule in neuron environment. J Phys Chem Lett 9:2009–2014. https://doi.org/10.1021/acs.jpclett.8b00324
Cantero MDR, Villa Etchegoyen C, Perez PL, Scarinci N, Cantiello HF (2018) Bundles of brain microtubules generate electrical oscillations. Sci Rep 8:11899. https://doi.org/10.1038/s41598-018-30453-2
Cantero MDR, Perez PL, Scarinci N, Cantiello HF (2019) Two-dimensional brain microtubule structures behave as memristive devices. Sci Rep 9:12398. https://doi.org/10.1038/s41598-019-48677-1
Kalra AP, Patel SD, Bhuiyan AF, Preto J, Scheuer KG, Mohammed U, Lewis JD, Rezania V, Shankar K, Tuszynski JA (2020) Investigation of the electrical properties of microtubule ensembles under cell-like conditions. Nanomaterials (Basel). https://doi.org/10.3390/nano10020265
Minoura I, Muto E (2006) Dielectric measurement of individual microtubules using the electroorientation method. Biophys J 90:3739–3748. https://doi.org/10.1529/biophysj.105.071324
Heimburg T, Jackson AD (2005) On soliton propagation in biomembranes and nerves. Proc Natl Acad Sci USA 102:9790–9795. https://doi.org/10.1073/pnas.0503823102
Leitner DM (2008) Energy flow in proteins. Annu Rev Phys Chem 59:233–259. https://doi.org/10.1146/annurev.physchem.59.032607.093606
Pokorny J, Foletti A, Kobilkova J, Jandova A, Vrba J, Vrba J Jr, Nedbalova M, Cocek A, Danani A, Tuszynski JA (2013) Biophysical insights into cancer transformation and treatment. Sci World J 2013:195028. https://doi.org/10.1155/2013/195028
Priel A, Ramos AJ, Tuszynski JA, Cantiello HF (2006) A biopolymer transistor: electrical amplification by microtubules. Biophys J 90:4639–4643. https://doi.org/10.1529/biophysj.105.078915
Tuszynski JA (2019) The bioelectric circuitry of the cell. In: Horner S, Noetscher M (eds) Brain and human body modeling Makarov. Springer, Cham, pp 195–208
Timmons JJ, Preto J, Tuszynski JA, Wong ET (2018) Tubulin’s response to external electric fields by molecular dynamics simulations. PLoS ONE 13:e0202141. https://doi.org/10.1371/journal.pone.0202141
Ma W, Korngreen A, Weil S, Cohen EB, Priel A, Kuzin L, Silberberg SD (2006) Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol 571:503–517. https://doi.org/10.1113/jphysiol.2005.103408
Uray IP, Uray K (2021) Mechanotransduction at the plasma membrane-cytoskeleton interface. Int J Mol Sci. https://doi.org/10.3390/ijms222111566
Priel A, Ramos AJ, Tuszynski JA, Cantiello HF (2008) Effect of calcium on electrical energy transfer by microtubules. J Biol Phys 34:475–485. https://doi.org/10.1007/s10867-008-9106-z
Vassilev P, Kanazirska M (1985) The role of cytoskeleton in the mechanisms of electric field effects and information transfer in cellular systems. Med Hypotheses 16:93–96. https://doi.org/10.1016/0306-9877(85)90065-9
Vassilev P, Kanazirska M, Tien HT (1985) Intermembrane linkage mediated by tubulin. Biochem Biophys Res Commun 126:559–565. https://doi.org/10.1016/0006-291x(85)90642-4
Kerr JP, Robison P, Shi G, Bogush AI, Kempema AM, Hexum JK, Becerra N, Harki DA, Martin SS, Raiteri R, Prosser BL, Ward CW (2015) Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun 6:8526. https://doi.org/10.1038/ncomms9526
Delphin C, Bouvier D, Seggio M, Couriol E, Saoudi Y, Denarier E, Bosc C, Valiron O, Bisbal M, Arnal I, Andrieux A (2012) MAP6-F is a temperature sensor that directly binds to and protects microtubules from cold-induced depolymerization. J Biol Chem 287:35127–35138. https://doi.org/10.1074/jbc.M112.398339
Lefevre J, Chernov KG, Joshi V, Delga S, Toma F, Pastre D, Curmi PA, Savarin P (2011) The C terminus of tubulin, a versatile partner for cationic molecules: binding of Tau, polyamines, and calcium. J Biol Chem 286:3065–3078. https://doi.org/10.1074/jbc.M110.144089
Solomon F (1977) Binding sites for calcium on tubulin. Biochemistry 16:358–363. https://doi.org/10.1021/bi00622a003
Regula CS, Pfeiffer JR, Berlin RD (1981) Microtubule assembly and disassembly at alkaline pH. J Cell Biol 89:45–53. https://doi.org/10.1083/jcb.89.1.45
Devillard L, Vandroux D, Tissier C, Brochot A, Voisin S, Rochette L, Athias P (2006) Tubulin ligands suggest a microtubule-NADPH oxidase relationship in postischemic cardiomyocytes. Eur J Pharmacol 548:64–73. https://doi.org/10.1016/j.ejphar.2006.08.004
Loncarek J, Bettencourt-Dias M (2018) Building the right centriole for each cell type. J Cell Biol 217:823–835. https://doi.org/10.1083/jcb.201704093
Janke C (2014) The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol 206:461–472. https://doi.org/10.1083/jcb.201406055
Wloga D, Gaertig J (2010) Post-translational modifications of microtubules. J Cell Sci 123:3447–3455. https://doi.org/10.1242/jcs.063727
Berling B, Wille H, Roll B, Mandelkow EM, Garner C, Mandelkow E (1994) Phosphorylation of microtubule-associated proteins MAP2a, b and MAP2c at Ser136 by proline-directed kinases in vivo and in vitro. Eur J Cell Biol 64:120–130
Zhu B, Zhang L, Creighton J, Alexeyev M, Strada SJ, Stevens T (2010) Protein kinase A phosphorylation of tau-serine 214 reorganizes microtubules and disrupts the endothelial cell barrier. Am J Physiol Lung Cell Mol Physiol 299:L493-501. https://doi.org/10.1152/ajplung.00431.2009
Alfaro-Aco R, Petry S (2015) Building the microtubule cytoskeleton piece by piece. J Biol Chem 290:17154–17162. https://doi.org/10.1074/jbc.R115.638452
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16:2166–2172. https://doi.org/10.1016/j.cub.2006.09.014
Arce CA, Casale CH, Barra HS (2008) Submembraneous microtubule cytoskeleton: regulation of ATPases by interaction with acetylated tubulin. FEBS J 275:4664–4674. https://doi.org/10.1111/j.1742-4658.2008.06615.x
Aguilar A, Becker L, Tedeschi T, Heller S, Iomini C, Nachury MV (2014) Alpha-tubulin K40 acetylation is required for contact inhibition of proliferation and cell-substrate adhesion. Mol Biol Cell 25:1854–1866. https://doi.org/10.1091/mbc.E13-10-0609
Shah N, Kumar S, Zaman N, Pan CC, Bloodworth JC, Lei W, Streicher JM, Hempel N, Mythreye K, Lee NY (2018) TAK1 activation of alpha-TAT1 and microtubule hyperacetylation control AKT signaling and cell growth. Nat Commun 9:1696. https://doi.org/10.1038/s41467-018-04121-y
Xu Z, Schaedel L, Portran D, Aguilar A, Gaillard J, Marinkovich MP, Thery M, Nachury MV (2017) Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356:328–332. https://doi.org/10.1126/science.aai8764
Tran AD, Marmo TP, Salam AA, Che S, Finkelstein E, Kabarriti R, Xenias HS, Mazitschek R, Hubbert C, Kawaguchi Y, Sheetz MP, Yao TP, Bulinski JC (2007) HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci 120:1469–1479. https://doi.org/10.1242/jcs.03431
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458. https://doi.org/10.1038/417455a
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21:6820–6831. https://doi.org/10.1093/emboj/cdf682
Rai A, Kapoor S, Singh S, Chatterji BP, Panda D (2015) Transcription factor NF-kappaB associates with microtubules and stimulates apoptosis in response to suppression of microtubule dynamics in MCF-7 cells. Biochem Pharmacol 93:277–289. https://doi.org/10.1016/j.bcp.2014.12.007
Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T (2000) p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol 2:709–717. https://doi.org/10.1038/35036335
Sunshine H, Iruela-Arispe ML (2017) Membrane lipids and cell signaling. Curr Opin Lipidol 28:408–413. https://doi.org/10.1097/MOL.0000000000000443
Freedman H, Rezania V, Priel A, Carpenter E, Noskov SY, Tuszynski JA (2010) Model of ionic currents through microtubule nanopores and the lumen. Phys Rev E Stat Nonlin Soft Matter Phys 81:051912. https://doi.org/10.1103/PhysRevE.81.051912
Kumar S, Boone K, Tuszynski J, Barclay P, Simon C (2016) Possible existence of optical communication channels in the brain. Sci Rep 6:36508. https://doi.org/10.1038/srep36508
Rahnama M, Tuszynski JA, Bokkon I, Cifra M, Sardar P, Salari V (2011) Emission of mitochondrial biophotons and their effect on electrical activity of membrane via microtubules. J Integr Neurosci 10:65–88. https://doi.org/10.1142/S0219635211002622
Kurian P, Obisesan TO, Craddock TJA (2017) Oxidative species-induced excitonic transport in tubulin aromatic networks: potential implications for neurodegenerative disease. J Photochem Photobiol B 175:109–124. https://doi.org/10.1016/j.jphotobiol.2017.08.033
Wagenknecht HA, Stemp ED, Barton JK (2000) DNA-Bound peptide radicals generated through DNA-mediated electron transport. Biochemistry 39:5483–5491. https://doi.org/10.1021/bi992897m
Cohen S, Popp FA (1997) Biophoton emission of the human body. J Photochem Photobiol B 40:187–189. https://doi.org/10.1016/s1011-1344(97)00050-x
Lin EM, Tierney DK, Stadtmauer EA (1993) Autologous bone marrow transplantation. A review of the principles and complications. Cancer Nurs 16:204–213
Arsenault ME, Zhao H, Purohit PK, Goldman YE, Bau HH (2007) Confinement and manipulation of actin filaments by electric fields. Biophys J 93:L42–L44. https://doi.org/10.1529/biophysj.107.114538
Schappi JM, Krbanjevic A, Rasenick MM (2014) Tubulin, actin and heterotrimeric G proteins: coordination of signaling and structure. Biochim Biophys Acta 1838:674–681. https://doi.org/10.1016/j.bbamem.2013.08.026
Siccardi M, Olagunju A, Simiele M, D’Avolio A, Calcagno A, Di Perri G, Bonora S, Owen A (2015) Class-specific relative genetic contribution for key antiretroviral drugs. J Antimicrob Chemother 70:3074–3079. https://doi.org/10.1093/jac/dkv207
Adamatzky A (2019) On discovering functions in actin filament automata. R Soc Open Sci 6:181198. https://doi.org/10.1098/rsos.181198
Priel A, Tuszynski JA, Woolf NJ (2010) Neural cytoskeleton capabilities for learning and memory. J Biol Phys 36:3–21. https://doi.org/10.1007/s10867-009-9153-0
Tarazi RC, Frohlich ED, Dustan HP (1968) Plasma volume in men with essential hypertension. N Engl J Med 278:762–765. https://doi.org/10.1056/NEJM196804042781404
Liu Y, Strumpfer J, Freddolino PL, Gruebele M, Schulten K (2012) Structural characterization of lambda-repressor folding from all-atom molecular dynamics simulations. J Phys Chem Lett 3:1117–1123. https://doi.org/10.1021/jz300017c
Hameroff SR (2004) A new theory of the origin of cancer: quantum coherent entanglement, centrioles, mitosis, and differentiation. Biosystems 77:119–136. https://doi.org/10.1016/j.biosystems.2004.04.006
Jang S, Hoyer S, Fleming G, Whaley KB (2014) Generalized master equation with non-Markovian multichromophoric Forster resonance energy transfer for modular exciton densities. Phys Rev Lett 113:188102. https://doi.org/10.1103/PhysRevLett.113.188102
Abasto DF, Mohseni M, Lloyd S, Zanardi P (2012) Exciton diffusion length in complex quantum systems: the effects of disorder and environmental fluctuations on symmetry-enhanced supertransfer. Philos Trans A Math Phys Eng Sci 370:3750–3770. https://doi.org/10.1098/rsta.2011.0213
Craddock TJ, Priel A, Tuszynski JA (2014) Keeping time: could quantum beating in microtubules be the basis for the neural synchrony related to consciousness? J Integr Neurosci 13:293–311. https://doi.org/10.1142/S0219635214400019
Hagan S, Hameroff SR, Tuszynski JA (2002) Quantum computation in brain microtubules: decoherence and biological feasibility. Phys Rev E Stat Nonlin Soft Matter Phys 65:061901. https://doi.org/10.1103/PhysRevE.65.061901
Craddock TJA, Beuachemin C, Tuszynski JA, and, (2009) Information processing mechanisms in microtubules at physiological temperature: model predictions for experimental tests. Biosystems 97:28–34
Hameroff S, Nip A, Porter M, Tuszynski J (2002) Conduction pathways in microtubules, biological quantum computation, and consciousness. Biosystems 64:149–168. https://doi.org/10.1016/s0303-2647(01)00183-6
Hackermuller L, Uttenthaler S, Hornberger K, Reiger E, Brezger B, Zeilinger A, Arndt M (2003) Wave nature of biomolecules and fluorofullerenes. Phys Rev Lett 91:090408. https://doi.org/10.1103/PhysRevLett.91.090408
Ouyang M, Awschalom DD (2003) Coherent spin transfer between molecularly bridged quantum dots. Science 301:1074–1078. https://doi.org/10.1126/science.1086963
Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R (2003) The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423:42–48. https://doi.org/10.1038/nature01581
Klein-Seetharaman J, Oikawa M, Grimshaw SB, Wirmer J, Duchardt E, Ueda T, Imoto T, Smith LJ, Dobson CM, Schwalbe H (2002) Long-range interactions within a nonnative protein. Science 295:1719–1722. https://doi.org/10.1126/science.1067680
Bahloul A, Meury J, Kern R, Garwood J, Guha S, Kohiyama M (1996) Co-ordination between membrane oriC sequestration factors and a chromosome partitioning protein, TolC (MukA). Mol Microbiol 22:275–282. https://doi.org/10.1046/j.1365-2958.1996.00106.x
Weber G (1970) Radicular pains in shoulder and arm. Schweiz Rundsch Med Prax 59:357–364 (In German)
Krasylenko Y, Yemets A, Blume Y (2013) Plant microtubules reorganization under the indirect UV-B exposure and during UV-B-induced programmed cell death. Plant Signal Behav 8:e24031. https://doi.org/10.4161/psb.24031
Calhoun TR, Ginsberg NS, Schlau-Cohen GS, Cheng YC, Ballottari M, Bassi R, Fleming GR (2009) Quantum coherence enabled determination of the energy landscape in light-harvesting complex II. J Phys Chem B 113:16291–16295. https://doi.org/10.1021/jp908300c
Collini E, Wong CY, Wilk KE, Curmi PM, Brumer P, Scholes GD (2010) Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 463:644–647. https://doi.org/10.1038/nature08811
Sardar PS, Maity SS, Das L, Ghosh S (2007) Luminescence studies of perturbation of tryptophan residues of tubulin in the complexes of tubulin with colchicine and colchicine analogues. Biochemistry 46:14544–14556. https://doi.org/10.1021/bi701412k
Bjork G, Pau S, Jacobson J, Yamamoto Y (1994) Wannier exciton superradiance in a quantum-well microcavity. Phys Rev B Condens Matter 50:17336–17348. https://doi.org/10.1103/physrevb.50.17336
Ilan Y (2016) Compounds of the sphingomyelin-ceramide-glycosphingolipid pathways as secondary messenger molecules: new targets for novel therapies for fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol 310:G1102–G1117. https://doi.org/10.1152/ajpgi.00095.2016
Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL (2010) Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA 107:9765–9770. https://doi.org/10.1073/pnas.0908771107
Shuvy M, Ben Ya’acov A, Zolotarov L, Lotan C, Ilan Y (2009) Beta glycosphingolipids suppress rank expression and inhibit natural killer T cell and CD8+ accumulation in alleviating aortic valve calcification. Int J Immunopathol Pharmacol 22:911–918. https://doi.org/10.1177/039463200902200406
Ben Ya’acov A, Lalazar G, Livovsky DM, Kanovich D, Axelrod E, Preston S, Schwarzmann G, Ilan Y (2009) Decreased STAT-1 phosphorylation by a thio analogue of beta-D-glucosylceramide is associated with altered NKT lymphocyte polarization. Mol Immunol 47:526–533. https://doi.org/10.1016/j.molimm.2009.07.030
Ilan Y (2009) Alpha versus beta: are we on the way to resolve the mystery as to which is the endogenous ligand for natural killer T cells? Clin Exp Immunol 158:300–307. https://doi.org/10.1111/j.1365-2249.2009.04030.x
Zhang W, Moritoki Y, Tsuneyama K, Yang GX, Ilan Y, Lian ZX, Gershwin ME (2009) Beta-glucosylceramide ameliorates liver inflammation in murine autoimmune cholangitis. Clin Exp Immunol 157:359–364. https://doi.org/10.1111/j.1365-2249.2009.03971.x
Lalazar G, Ben Ya’acov A, Livovsky DM, El Haj M, Pappo O, Preston S, Zolotarov L, Ilan Y (2009) Beta-glycoglycosphingolipid-induced alterations of the STAT signaling pathways are dependent on CD1d and the lipid raft protein flotillin-2. Am J Pathol 174:1390–1399. https://doi.org/10.2353/ajpath.2009.080841
Prentice-Mott HV, Meroz Y, Carlson A, Levine MA, Davidson MW, Irimia D, Charras GT, Mahadevan L, Shah JV (2016) Directional memory arises from long-lived cytoskeletal asymmetries in polarized chemotactic cells. Proc Natl Acad Sci USA 113:1267–1272. https://doi.org/10.1073/pnas.1513289113
Cocchi M, Bernroider G, Rasenick M, Tonello L, Gabrielli F, Tuszynski JA (2017) Document of Trapani on animal consciousness and quantum brain function: a hypothesis. J Integr Neurosci 16:S99–S103. https://doi.org/10.3233/JIN-170070
Jibu MYK (1995) Quantum brain dynamics and consciousness: an introduction, advances in consciousness research. John Benjamins Publishing Company, Philadelphia, p 3
Stuart CI, Takahashi Y, Umezawa H (1978) On the stability and non-local properties of memory. J Theor Biol 71:605–618. https://doi.org/10.1016/0022-5193(78)90327-2
Keppler J (2013) A new perspective on the functioning of the brain and the mechanisms behind conscious processes. Front Psychol 4:242. https://doi.org/10.3389/fpsyg.2013.00242
Penrose R (1989) The emperor’s new mind. Oxford University Press, Oxford
Penrose R (1994) Shadows of the mind. Oxford University Press, Oxford
Photos E, Busemeyer J (2013) Can quantum probability provide a new direction for cognitive modeling? Behav Brain Sci 36:255–327
Barlow PW (2015) The natural history of consciousness, and the question of whether plants are conscious, in relation to the Hameroff-Penrose quantum-physical “Orch OR” theory of universal consciousness. Commun Integr Biol 8:e1041696. https://doi.org/10.1080/19420889.2015.1041696
Brown JAT (1997) Dipole interactions in axonal microtubules as a mechanism of signal propagation. Phys Rev E 56:5834–5840
Woolf NJ, Craddock TJA, Friesen DE, Tuszynski JA (2010) Neuropsychiatric illness: a case for impaired neuroplasticity and possible quantum processing derailment in microtubules. Neuroquantology 8:13–28
Tuszynski JA (2014) The need for a physical basis of cognitive process: comment on “Consciousness in the universe. A review of the ‘Orch OR’ theory” by Hameroff and Penrose. Phys Life Rev 11:79–80. https://doi.org/10.1016/j.plrev.2013.10.009 (discussion 94-100)
Engelbrecht J, Peets T, Tamm K (2018) Electromechanical coupling of waves in nerve fibres. Biomech Model Mechanobiol 17:1771–1783. https://doi.org/10.1007/s10237-018-1055-2
Fongang Achu G, Moukam Kakmeni FM, Dikande AM (2018) Breathing pulses in the damped-soliton model for nerves. Phys Rev E 97:012211. https://doi.org/10.1103/PhysRevE.97.012211
Craddock TJ, Tuszynski JA, Hameroff S (2012) Cytoskeletal signaling: is memory encoded in microtubule lattices by CaMKII phosphorylation? PLoS Comput Biol 8:e1002421. https://doi.org/10.1371/journal.pcbi.1002421
Abbott LF, Regehr WG (2004) Synaptic computation. Nature 431:796–803. https://doi.org/10.1038/nature03010
Craddock TJ, St George M, Freedman H, Barakat KH, Damaraju S, Hameroff S, Tuszynski JA (2012) Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: implications for side effects of general anesthesia. PLoS ONE 7:e37251. https://doi.org/10.1371/journal.pone.0037251
Hameroff S (2012) Quantum brain biology complements neuronal assembly approaches to consciousness: comment on “Consciousness, biology and quantum hypotheses” by Baars and Edelman. Phys Life Rev 9:303–305. https://doi.org/10.1016/j.plrev.2012.07.002 (discussion 306-7)
Hameroff S (2010) The “conscious pilot”-dendritic synchrony moves through the brain to mediate consciousness. J Biol Phys 36:71–93. https://doi.org/10.1007/s10867-009-9148-x
Beer S, Alexandre K, Pecoud S, Ruiz J (2006) Gestational diabetes: what follow-up after delivery? Rev Med Suisse 2:1468–1472
Isshiki K, Hirase T, Matsuda S, Miyamoto K, Tsuji A, Yuasa K (2015) Death-associated protein kinase 2 mediates nocodazole-induced apoptosis through interaction with tubulin. Biochem Biophys Res Commun 468:113–118. https://doi.org/10.1016/j.bbrc.2015.10.151
Penrose R (2011) Uncertainty in quantum mechanics: faith or fantasy? Philos Trans A Math Phys Eng Sci 369:4864–4890. https://doi.org/10.1098/rsta.2011.0179
Hameroff S (1998) Anesthesia, consciousness and hydrophobic pockets–a unitary quantum hypothesis of anesthetic action. Toxicol Lett 100–101:31–39. https://doi.org/10.1016/s0378-4274(98)00162-3
Hameroff SR (1998) `Funda-Mentality’: is the conscious mind subtly linked to a basic level of the universe? Trends Cogn Sci 2:119–124. https://doi.org/10.1016/s1364-6613(98)01157-7
Penrose R, Hameroff SR (1996) Conscious events as orchestrate dspace-time selections. J Conscious Stud 3:36–53
Pan JZ, Xi J, Tobias JW, Eckenhoff MF, Eckenhoff RG (2007) Halothane binding proteome in human brain cortex. J Proteome Res 6:582–592. https://doi.org/10.1021/pr060311u
Xi J, Liu R, Asbury GR, Eckenhoff MF, Eckenhoff RG (2004) Inhalational anesthetic-binding proteins in rat neuronal membranes. J Biol Chem 279:19628–19633. https://doi.org/10.1074/jbc.M313864200
Butts CA, Xi J, Brannigan G, Saad AA, Venkatachalan SP, Pearce RA, Klein ML, Eckenhoff RG, Dmochowski IJ (2009) Identification of a fluorescent general anesthetic, 1-aminoanthracene. Proc Natl Acad Sci USA 106:6501–6506. https://doi.org/10.1073/pnas.0810590106
Emerson DJ, Weiser BP, Psonis J, Liao Z, Taratula O, Fiamengo A, Wang X, Sugasawa K, Smith AB 3rd, Eckenhoff RG, Dmochowski IJ (2013) Direct modulation of microtubule stability contributes to anthracene general anesthesia. J Am Chem Soc 135:5389–5398. https://doi.org/10.1021/ja311171u
Craddock TJ, Hameroff SR, Ayoub AT, Klobukowski M, Tuszynski JA (2015) Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Curr Top Med Chem 15:523–533. https://doi.org/10.2174/1568026615666150225104543
Meyer H (1901) Theorie Der Alkolnarkose. Zur Naunyn Schmied Arch Exp Path Pharmakol 46:338–346
Overton E (1901) Studien Ueber Die Narkose. Jena, Gustav Fischer (In German)
Craddock TJA, Kurian P, Preto J, Sahu K, Hameroff SR, Klobukowski M, Tuszynski JA (2017) Anesthetic alterations of collective terahertz oscillations in tubulin correlate with clinical potency: implications for anesthetic action and post-operative cognitive dysfunction. Sci Rep 7:9877. https://doi.org/10.1038/s41598-017-09992-7
Allison AC, Nunn JF (1968) Effects of general anaesthetics on microtubules: a possible mechanism of anaesthesia. Lancet 2:1326–1329. https://doi.org/10.1016/s0140-6736(68)91821-7
Stroppolo ME, Falconi M, Caccuri AM, Desideri A (2001) Superefficient enzymes. Cell Mol Life Sci 58:1451–1460. https://doi.org/10.1007/PL00000788
Ambrosetti A, Ferri N, DiStasio RA Jr, Tkatchenko A (2016) Wavelike charge density fluctuations and van der Waals interactions at the nanoscale. Science 351:1171–1176. https://doi.org/10.1126/science.aae0509
Dobson JF (2014) Beyond pairwise additivity in London dispersion interactions. Int J Quantum Chem 114:1157–1161
Kurian P, Capolupo A, Craddock TJA, Vitiello G (2018) Water-mediated correlations in DNA-enzyme interactions. Phys Lett A 382:33–43. https://doi.org/10.1016/j.physleta.2017.10.038
Kurian P, Dunston G, Lindesay J (2016) How quantum entanglement in DNA synchronizes double-strand breakage by type II restriction endonucleases. J Theor Biol 391:102–112. https://doi.org/10.1016/j.jtbi.2015.11.018
Honore S, Hubert F, Tournus M, White D (2019) A growth-fragmentation approach for modeling microtubule dynamic instability. Bull Math Biol 81:722–758. https://doi.org/10.1007/s11538-018-0531-2
Florian S, Mitchison TJ (2016) Anti-microtubule drugs. Methods Mol Biol 1413:403–421. https://doi.org/10.1007/978-1-4939-3542-0_25
McLoughlin EC, O’Boyle NM (2020) Colchicine-binding site inhibitors from chemistry to clinic: a review. Pharmaceuticals (Basel). https://doi.org/10.3390/ph13010008
Schenone AL, Menon V (2018) Colchicine in pericardial disease: from the underlying biology and clinical benefits to the drug-drug interactions in cardiovascular medicine. Curr Cardiol Rep 20:62. https://doi.org/10.1007/s11886-018-1008-5
Robinson KP, Chan JJ (2018) Colchicine in dermatology: a review. Australas J Dermatol 59:278–285. https://doi.org/10.1111/ajd.12795
Pascart T, Richette P (2018) Colchicine in gout: an update. Curr Pharm Des 24:684–689. https://doi.org/10.2174/1381612824999180115103951
Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH (2018) Update on colchicine, 2017. Rheumatology (Oxford) 57:i4–i11. https://doi.org/10.1093/rheumatology/kex453
Majcher U, Klejborowska G, Kaik M, Maj E, Wietrzyk J, Moshari M, Preto J, Tuszynski JA, Huczynski A (2018) Synthesis and biological evaluation of novel triple-modified colchicine derivatives as potent tubulin-targeting anticancer agents. Cells. https://doi.org/10.3390/cells7110216
Gigant B, Cormier A, Dorleans A, Ravelli RB, Knossow M (2009) Microtubule-destabilizing agents: structural and mechanistic insights from the interaction of colchicine and vinblastine with tubulin. Top Curr Chem 286:259–278. https://doi.org/10.1007/128_2008_11
Arnst KE, Wang Y, Lei ZN, Hwang DJ, Kumar G, Ma D, Parke DN, Chen Q, Yang J, White SW, Seagroves TN, Chen ZS, Miller DD, Li W (2019) Colchicine binding site agent DJ95 overcomes drug resistance and exhibits antitumor efficacy. Mol Pharmacol 96:73–89. https://doi.org/10.1124/mol.118.114801
Erden A, Batu ED, Sari A, Sonmez HE, Armagan B, Demir S, Firat E, Bilginer Y, Bilgen SA, Karadag O, Kalyoncu U, Kiraz S, Ertenli I, Ozen S, Akdogan A (2018) Which definition should be used to determine colchicine resistance among patients with familial Mediterranean fever? Clin Exp Rheumatol 36:97–102
Corsia A, Georgin-Lavialle S, Hentgen V, Hachulla E, Grateau G, Faye A, Quartier P, Rossi-Semerano L, Kone-Paut I (2017) A survey of resistance to colchicine treatment for French patients with familial Mediterranean fever. Orphanet J Rare Dis 12:54. https://doi.org/10.1186/s13023-017-0609-1
Ozen S, Kone-Paut I, Gul A (2017) Colchicine resistance and intolerance in familial mediterranean fever: definition, causes, and alternative treatments. Semin Arthritis Rheum 47:115–120. https://doi.org/10.1016/j.semarthrit.2017.03.006
Ozer I, Mete T, Turkeli Sezer O, Kolbasi Ozgen G, Kucuk GO, Kaya C, Kilic Kan E, Duman G, Ozturk Kurt HP (2015) Association between colchicine resistance and vitamin D in familial Mediterranean fever. Ren Fail 37:1122–1125. https://doi.org/10.3109/0886022X.2015.1056064
Spasevska I, Ayoub AT, Winter P, Preto J, Wong GK, Dumontet C, Tuszynski JA (2017) Modeling the colchicum autumnale tubulin and a comparison of its interaction with colchicine to human tubulin. Int J Mol Sci. https://doi.org/10.3390/ijms18081676
Sahakyan H, Abelyan N, Arakelov V, Arakelov G, Nazaryan K (2019) In silico study of colchicine resistance molecular mechanisms caused by tubulin structural polymorphism. PLoS ONE 14:e0221532. https://doi.org/10.1371/journal.pone.0221532
Omma A, Sandikci SC, Kucuksahin O, Alisik M, Erel O (2017) Can the thiol/disulfide imbalance be a predictor of colchicine resistance in familial mediterranean fever? J Korean Med Sci 32:1588–1594. https://doi.org/10.3346/jkms.2017.32.10.1588
Rai A, Kapoor S, Naaz A, Kumar Santra M, Panda D (2017) Enhanced stability of microtubules contributes in the development of colchicine resistance in MCF-7 cells. Biochem Pharmacol 132:38–47. https://doi.org/10.1016/j.bcp.2017.02.018
Gangjee A, Zhao Y, Lin L, Raghavan S, Roberts EG, Risinger AL, Hamel E, Mooberry SL (2010) Synthesis and discovery of water-soluble microtubule targeting agents that bind to the colchicine site on tubulin and circumvent Pgp mediated resistance. J Med Chem 53:8116–8128. https://doi.org/10.1021/jm101010n
Lin MS, Hong TM, Chou TH, Yang SC, Chung WC, Weng CW, Tsai ML, Cheng TR, Chen JJW, Lee TC, Wong CH, Chein RJ, Yang PC (2019) 4(1H)-quinolone derivatives overcome acquired resistance to anti-microtubule agents by targeting the colchicine site of beta-tubulin. Eur J Med Chem 181:111584. https://doi.org/10.1016/j.ejmech.2019.111584
Bai Z, Gao M, Zhang H, Guan Q, Xu J, Li Y, Qi H, Li Z, Zuo D, Zhang W, Wu Y (2017) BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe. Cancer Lett 402:81–92. https://doi.org/10.1016/j.canlet.2017.05.016
Arnst KE, Wang Y, Hwang DJ, Xue Y, Costello T, Hamilton D, Chen Q, Yang J, Park F, Dalton JT, Miller DD, Li W (2018) A potent, metabolically stable tubulin inhibitor targets the colchicine binding site and overcomes taxane resistance. Cancer Res 78:265–277. https://doi.org/10.1158/0008-5472.CAN-17-0577
Kim MS, Cho J (2009) Physics. Teleporting a quantum state to distant matter. Science 323:469–470
Wiersma DS (2010) Physics. Random quantum networks. Science 327:1333–1334. https://doi.org/10.1126/science.1187084327/5971/1333
Bernien H, Hensen B, Pfaff W, Koolstra G, Blok MS, Robledo L, Taminiau TH, Markham M, Twitchen DJ, Childress L, Hanson R (2013) Heralded entanglement between solid-state qubits separated by three metres. Nature 497:86–90. https://doi.org/10.1038/nature12016nature12016[pii]
Ma XS, Herbst T, Scheidl T, Wang D, Kropatschek S, Naylor W, Wittmann B, Mech A, Kofler J, Anisimova E, Makarov V, Jennewein T, Ursin R, Zeilinger A (2012) Quantum teleportation over 143 kilometres using active feed-forward. Nature 489:269–273. https://doi.org/10.1038/nature11472
Lamas-Linares A, Howell JC, Bouwmeester D (2001) Stimulated emission of polarization-entangled photons. Nature 412:887–890
Pan JW, Bouwmeester D, Daniell M, Weinfurter H, Zeilinger A (2000) Experimental test of quantum nonlocality in three-photon Greenberger-Horne-Zeilinger entanglement. Nature 403:515–519
Hardy L (1992) Quantum mechanics, local realistic theories, and Lorentz-invariant realistic theories. Phys Rev Lett 68:2981–2984
Olmschenk S, Matsukevich DN, Maunz P, Hayes D, Duan LM, Monroe C (2009) Quantum teleportation between distant matter qubits. Science 323:486–489
Sapienza L, Thyrrestrup H, Stobbe S, Garcia PD, Smolka S, Lodahl P (2010) Cavity quantum electrodynamics with Anderson-localized modes. Science 327:1352–1355
Ilan Y (2018) Mehtos and system for modulating physiological states between biological entities. Patent US 2018/0328917 A1
Shabat Y, Lichtenstein Y, Ilan Y (2021) Short-term cohousing of sick with healthy or treated mice alleviates the inflammatory response and liver damage. Inflammation 44:518–525. https://doi.org/10.1007/s10753-020-01348-0
Ilan Y (2019) Immune rebalancing by oral immunotherapy: a novel method for getting the immune system back on track. J Leukoc Biol 105:463–472. https://doi.org/10.1002/JLB.5RU0718-276RR
Ilan Y (2016) Oral immune therapy: targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease. Clin Transl Immunology 5:e60. https://doi.org/10.1038/cti.2015.47
Ilan Y (2016) Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression. Aliment Pharmacol Ther 44:1168–1182. https://doi.org/10.1111/apt.13833
Ilan Y, Shailubhai K, Sanyal A (2018) Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH. Clin Exp Immunol 193:275–283. https://doi.org/10.1111/cei.13159
Almon E, Khoury T, Drori A, Gingis-Velitski S, Alon S, Chertkoff R, Mushkat M, Shaaltiel Y, Ilan Y (2017) An oral administration of a recombinant anti-TNF fusion protein is biologically active in the gut promoting regulatory T cells: results of a phase I clinical trial using a novel oral anti-TNF alpha-based therapy. J Immunol Methods 446:21–29. https://doi.org/10.1016/j.jim.2017.03.023
Lalazar G, Zigmond E, Weksler-Zangen S, Ya’acov AB, Levy MS, Hemed N, Raz I, Ilan Y (2017) Oral administration of beta-glucosylceramide for the treatment of insulin resistance and nonalcoholic steatohepatitis: results of a double-blind, placebo-controlled trial. J Med Food 20:458–464. https://doi.org/10.1089/jmf.2016.3753
Ilan Y, Gingis-Velitski S, Ben Ya’aco A, Shabbat Y, Zolotarov L, Almon E, Shaaltiel Y (2017) A plant cell-expressed recombinant anti-TNF fusion protein is biologically active in the gut and alleviates immune-mediated hepatitis and colitis. Immunobiology 222:544–551. https://doi.org/10.1016/j.imbio.2016.11.001
Ilan Y, Ben Ya’acov A, Shabbat Y, Gingis-Velitski S, Almon E, Shaaltiel Y (2016) Oral administration of a non-absorbable plant cell-expressed recombinant anti-TNF fusion protein induces immunomodulatory effects and alleviates nonalcoholic steatohepatitis. World J Gastroenterol 22:8760–8769. https://doi.org/10.3748/wjg.v22.i39.8760
Israeli E, Zigmond E, Lalazar G, Klein A, Hemed N, Goldin E, Ilan Y (2015) Oral mixture of autologous colon-extracted proteins for the Crohn’s disease: A double-blind trial. World J Gastroenterol 21:5685–5694. https://doi.org/10.3748/wjg.v21.i18.5685
Lalazar G, Mizrahi M, Turgeman I, Adar T, Ben Ya’acov A, Shabat Y, Nimer A, Hemed N, Zolotarovya L, Lichtenstein Y, Lisovoder N, Samira S, Shalit I, Ellis R, Ilan Y (2015) Oral administration of OKT3 MAb to patients with NASH, promotes regulatory T-cell induction, and alleviates insulin resistance: results of a phase iia blinded placebo-controlled trial. J Clin Immunol 35:399–407. https://doi.org/10.1007/s10875-015-0160-6
Israeli E, Goldin E, Fishman S, Konikoff F, Lavy A, Chowers Y, Melzer E, Lahat A, Mahamid M, Shirin H, Nussinson E, Segol O, Ya’acov AB, Shabbat Y, Ilan Y (2015) Oral administration of non-absorbable delayed release 6-mercaptopurine is locally active in the gut, exerts a systemic immune effect and alleviates Crohn’s disease with low rate of side effects: results of double blind Phase II clinical trial. Clin Exp Immunol 181:362–372. https://doi.org/10.1111/cei.12640
Dush MK, Nascone-Yoder NM (2013) Jun N-terminal kinase maintains tissue integrity during cell rearrangement in the gut. Development (Cambridge, England) 140:1457–1466. https://doi.org/10.1242/dev.086850
Zhou X, Xiao C, Li Y, Shang Y, Yin D, Li S, Xiang B, Lu R, Ji Y, Wu Y, Meng W, Zhu H, Liu J, Hu H, Mo X, Xu H (2018) Mid1ip1b modulates apical reorientation of non-centrosomal microtubule organizing center in epithelial cells. J Genet Genomics = Yi chuan xue bao 45:433–442. https://doi.org/10.1016/j.jgg.2018.08.001
Höfer D, Jöns T, Kraemer J, Drenckhahn D (1998) From cytoskeleton to polarity and chemoreception in the gut epithelium. Ann N Y Acad Sci 859:75–84. https://doi.org/10.1111/j.1749-6632.1998.tb11112.x
Shi C, Li H, Qu X, Huang L, Kong C, Qin H, Sun Z, Yan X (2019) High fat diet exacerbates intestinal barrier dysfunction and changes gut microbiota in intestinal-specific ACF7 knockout mice. Biomed Pharmacother 110:537–545. https://doi.org/10.1016/j.biopha.2018.11.100
Mimori-Kiyosue Y, Shiina N, Tsukita S (2000) Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol 148:505–518. https://doi.org/10.1083/jcb.148.3.505
Kilner J, Corfe BM, McAuley MT, Wilkinson SJ (2016) A deterministic oscillatory model of microtubule growth and shrinkage for differential actions of short chain fatty acids. Mol Biosyst 12:93–101. https://doi.org/10.1039/c5mb00211g
Gerding DN, Johnson S, Rupnik M, Aktories K (2014) Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes 5:15–27. https://doi.org/10.4161/gmic.26854
Lu LF, Kim DH, Lee IH, Hong J, Zhang P, Yoon IN, Hwang JS, Kim H (2016) Potassium acetate blocks clostridium difficile toxin A-induced microtubule disassembly by directly inhibiting histone deacetylase 6, thereby ameliorating inflammatory responses in the gut. J Microbiol Biotechnol 26:693–699. https://doi.org/10.4014/jmb.1511.11063
Kopecko DJ, Hu L, Zaal KJ (2001) Campylobacter jejuni–microtubule-dependent invasion. Trends Microbiol 9:389–396. https://doi.org/10.1016/s0966-842x(01)02107-2
Hsu C-R, Pan Y-J, Liu J-Y, Chen C-T, Lin T-L, Wang J-T (2015) Klebsiella pneumoniae translocates across the intestinal epithelium via Rho GTPase- and phosphatidylinositol 3-kinase/Akt-dependent cell invasion. Infect Immun 83:769–779. https://doi.org/10.1128/IAI.02345-14
Kim J-H, Yamamoto T, Lee J, Yashiro T, Hamada T, Hayashi S, Kadowaki M (2014) CGRP, a neurotransmitter of enteric sensory neurons, contributes to the development of food allergy due to the augmentation of microtubule reorganization in mucosal mast cells. Biomed Res (Tokyo, Japan) 35:285–293. https://doi.org/10.2220/biomedres.35.285
Nazli A, Wang A, Steen O, Prescott D, Lu J, Perdue MH, Soderholm JD, Sherman PM, McKay DM (2006) Enterocyte cytoskeleton changes are crucial for enhanced translocation of nonpathogenic Escherichia coli across metabolically stressed gut epithelia. Infect Immun 74:192–201. https://doi.org/10.1128/IAI.74.1.192-201.2006
Ilan YBI T (2019) A subject sepcific system and method for prevention of body adapatation for chronic treatment of disease. WO 2019/008571 Al. https://patents.google.com/patent/WO2019008571A1/en
Tonello L, Gashi B, Scuotto A, Cappello G, Cocchi M, Gabrielli F, Tuszynski JA (2018) The gastrointestinal-brain axis in humans as an evolutionary advance of the root-leaf axis in plants: a hypothesis linking quantum effects of light on serotonin and auxin. J Integr Neurosci 17:177–183. https://doi.org/10.31083/JIN-170048
Azmitia EC (2007) Serotonin and brain: evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol 77:31–56. https://doi.org/10.1016/S0074-7742(06)77002-7
Baluska F, Mancuso S (2016) Vision in plants via plant-specific ocelli? Trends Plant Sci 21:727–730. https://doi.org/10.1016/j.tplants.2016.07.008
Grass F, Klima H, Kasper S (2004) Biophotons, microtubules and CNS, is our brain a “holographic computer”? Med Hypotheses 62:169–172. https://doi.org/10.1016/S0306-9877(03)00308-6
Tonello L, Cocchi M, Gabrielli F, Tuszynski JA (2015) On the possible quantum role of serotonin in consciousness. J Integr Neurosci 14:295–308. https://doi.org/10.1142/S021963521550017X
Franklin KA, Whitelam GC (2007) Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat Genet 39:1410–1413. https://doi.org/10.1038/ng.2007.3
Halliday KJ, Martinez-Garcia JF, Josse EM (2009) Integration of light and auxin signaling. Cold Spring Harb Perspect Biol 1:a001586. https://doi.org/10.1101/cshperspect.a001586
Fidalgo S, Ivanov DK, Wood SH (2013) Serotonin: from top to bottom. Biogerontology 14:21–45. https://doi.org/10.1007/s10522-012-9406-3
De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Backhed F (2018) Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA 115:6458–6463. https://doi.org/10.1073/pnas.1720017115
Greig CJ, Gandotra N, Tackett JJ, Bamdad MC, Cowles RA (2016) Enhanced serotonin signaling increases intestinal neuroplasticity. J Surg Res 206:151–158. https://doi.org/10.1016/j.jss.2016.07.021
Fernandez SP, Muzerelle A, Scotto-Lomassese S, Barik J, Gruart A, Delgado-Garcia JM, Gaspar P (2017) Constitutive and acquired serotonin deficiency alters memory and hippocampal synaptic plasticity. Neuropsychopharmacology 42:512–523. https://doi.org/10.1038/npp.2016.134
Ge X, Pan J, Liu Y, Wang H, Zhou W, Wang X (2018) Intestinal crosstalk between microbiota and serotonin and its impact on gut motility. Curr Pharm Biotechnol 19:190–195. https://doi.org/10.2174/1389201019666180528094202
Smith TK, Koh SD (2017) A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am J Physiol Gastrointest Liver Physiol 312:G1–G14. https://doi.org/10.1152/ajpgi.00337.2016
Stasi C, Sadalla S, Milani S (2019) The relationship between the serotonin metabolism, gut-microbiota and the gut-brain axis. Curr Drug Metab 20:646–655. https://doi.org/10.2174/1389200220666190725115503
Dong Y, Han Y, Wang Z, Qin Z, Yang C, Cao J, Chen Y (2017) Role of serotonin on the intestinal mucosal immune response to stress-induced diarrhea in weaning mice. BMC Gastroenterol 17:82. https://doi.org/10.1186/s12876-017-0634-5
Wirth A, Holst K, Ponimaskin E (2017) How serotonin receptors regulate morphogenic signalling in neurons. Prog Neurobiol 151:35–56. https://doi.org/10.1016/j.pneurobio.2016.03.007
Vinuelas J, Kaneko G, Coulon A, Beslon G, Gandrillon O (2012) Towards experimental manipulation of stochasticity in gene expression. Prog Biophys Mol Biol 110:44–53. https://doi.org/10.1016/j.pbiomolbio.2012.04.010
Boettiger AN (2013) Analytic approaches to stochastic gene expression in multicellular systems. Biophys J 105:2629–2640. https://doi.org/10.1016/j.bpj.2013.10.033
Eldar A, Elowitz MB (2010) Functional roles for noise in genetic circuits. Nature 467:167–173. https://doi.org/10.1038/nature09326
Phillips NE, Manning C, Papalopulu N, Rattray M (2017) Identifying stochastic oscillations in single-cell live imaging time series using Gaussian processes. PLoS Comput Biol 13:e1005479. https://doi.org/10.1371/journal.pcbi.1005479
Wang G, Sun S, Zhang Z (2016) Randomness in sequence evolution increases over time. PLoS ONE 11:e0155935. https://doi.org/10.1371/journal.pone.0155935
Streit A, Sommer RJ (2010) Genetics: random expression goes binary. Nature 463:891–892. https://doi.org/10.1038/463891a
Raj A, Rifkin SA, Andersen E, van Oudenaarden A (2010) Variability in gene expression underlies incomplete penetrance. Nature 463:913–918. https://doi.org/10.1038/nature08781
Ilan Y (2020) Overcoming compensatory mechanisms toward chronic drug administration to ensure long-term, sustainable beneficial effects. Mol Ther Methods Clin Dev 18:335–344. https://doi.org/10.1016/j.omtm.2020.06.006
Gilpin WSBM, Prakash M (2020) The multiscale physics of cilia and flagella. Nat Rev Phys. https://doi.org/10.1038/s42254-019-0129-0
Han J, Peskin CS (2018) Spontaneous oscillation and fluid-structure interaction of cilia. Proc Natl Acad Sci USA 115:4417–4422. https://doi.org/10.1073/pnas.1712042115
Ilan Y (2020) Order through disorder: the characteristic variability of systems. Front Cell Dev Biol 8:186. https://doi.org/10.3389/fcell.2020.00186
Smith DJ, Gaffney EA, Blake JR (2007) A viscoelastic traction layer model of muco-ciliary transport. Bull Math Biol 69:289–327. https://doi.org/10.1007/s11538-005-9036-x
Clifford JC, Rapicavoli JN, Roper MC (2013) A rhamnose-rich O-antigen mediates adhesion, virulence, and host colonization for the xylem-limited phytopathogen Xylella fastidiosa. Mol Plant Microbe Interact 26:676–685. https://doi.org/10.1094/MPMI-12-12-0283-R
Orme BA, Otto SR, Blake JR (2001) Enhanced efficiency of feeding and mixing due to chaotic flow patterns around choanoflagellates. IMA J Math Appl Med Biol 18:293–325
Kirkegaard JB, Marron AO, Goldstein RE (2016) Motility of colonial choanoflagellates and the statistics of aggregate random walkers. Phys Rev Lett 116:038102. https://doi.org/10.1103/PhysRevLett.116.038102
El-Haj M, Kanovitch D, Ilan Y (2019) Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: a novel platform for designing personalized immunotherapies. Immunol Res 67:337–347. https://doi.org/10.1007/s12026-019-09101-y
Ilan Y (2019) Why targeting the microbiome is not so successful: can randomness overcome the adaptation that occurs following gut manipulation? Clin Exp Gastroenterol 12:209–217. https://doi.org/10.2147/CEG.S203823
Ilan Y, Spigelman Z (2020) Establishing patient-tailored variability-based paradigms for anti-cancer therapy: using the inherent trajectories which underlie cancer for overcoming drug resistance. Cancer Treat Res Commun 25:100240. https://doi.org/10.1016/j.ctarc.2020.100240
Kessler A, Weksler-Zangen S, Ilan Y (2020) Role of the immune system and the circadian rhythm in the pathogenesis of chronic pancreatitis: establishing a personalized signature for improving the effect of immunotherapies for chronic pancreatitis. Pancreas 49:1024–1032. https://doi.org/10.1097/MPA.0000000000001626
Kolben Y, Weksler-Zangen S, Ilan Y (2021) Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: implementing a personalized signature-based platform for chronotherapy. Obes Rev 22:e13108. https://doi.org/10.1111/obr.13108
Potruch A, Khoury ST, Ilan Y (2020) The role of chronobiology in drug-resistance epilepsy: the potential use of a variability and chronotherapy-based individualized platform for improving the response to anti-seizure drugs. Seizure 80:201–211. https://doi.org/10.1016/j.seizure.2020.06.032
Forkosh E, Kenig A, Ilan Y (2020) Introducing variability in targeting the microtubules: review of current mechanisms and future directions in colchicine therapy. Pharmacol Res Perspect 8:e00616. https://doi.org/10.1002/prp2.616
Gelman R, Bayatra A, Kessler A, Schwartz A, Ilan Y (2020) Targeting SARS-CoV-2 receptors as a means for reducing infectivity and improving antiviral and immune response: an algorithm-based method for overcoming resistance to antiviral agents. Emerg Microbes Infect 9:1397–1406. https://doi.org/10.1080/22221751.2020.1776161
Kenig A, Kolben Y, Asleh R, Amir O, Ilan Y (2021) Improving diuretic response in heart failure by implementing a patient-tailored variability and chronotherapy-guided algorithm. Front Cardiovasc Med 8:695547. https://doi.org/10.3389/fcvm.2021.695547
Ishay Y, Kolben Y, Kessler A, Ilan Y (2021) Role of circadian rhythm and autonomic nervous system in liver function: a hypothetical basis for improving the management of hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 321:G400–G412. https://doi.org/10.1152/ajpgi.00186.2021
Hurvitz N, Azmanov H, Kesler A, Ilan Y (2021) Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur J Hum Genet. https://doi.org/10.1038/s41431-021-00928-4
Azmanov H, Ross EL, Ilan Y (2021) Establishment of an individualized chronotherapy, autonomic nervous system, and variability-based dynamic platform for overcoming the loss of response to analgesics. Pain Phys 24:243–252
Khoury T, Ilan Y (2021) Platform introducing individually tailored variability in nerve stimulations and dietary regimen to prevent weight regain following weight loss in patients with obesity. Obes Res Clin Pract 15:114–123. https://doi.org/10.1016/j.orcp.2021.02.003
Ilan Y (2021) Improving global healthcare and reducing costs using second-generation artificial intelligence-based digital pills: a market disruptor. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18020811
Isahy Y, Ilan Y (2021) Improving the long-term response to antidepressants by establishing an individualized platform based on variability and chronotherapy. Int J Clin Pharmacol Ther 59:768–774. https://doi.org/10.5414/CP204000
Ishay Y, Potruch A, Schwartz A, Berg M, Jamil K, Agus S, Ilan Y (2021) A digital health platform for assisting the diagnosis and monitoring of COVID-19 progression: an adjuvant approach for augmenting the antiviral response and mitigating the immune-mediated target organ damage. Biomed Pharmacother 143:112228. https://doi.org/10.1016/j.biopha.2021.112228
Hurvitz N, Azmanov H, Kesler A, Ilan Y (2021) Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur J Hum Genet 29:1485–1490. https://doi.org/10.1038/s41431-021-00928-4
Ilan Y (2021) Digital medical cannabis as market differentiator: second-generation artificial intelligence systems to improve response. Front Med (Lausanne) 8:788777. https://doi.org/10.3389/fmed.2021.788777
Gelman R, Berg M, Ilan Y (2022) A subject-tailored variability-based platform for overcoming the plateau effect in sports training: a narrative review. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph19031722
Azmanov H, Bayatra A, Ilan Y (2022) Digital analgesic comprising a second-generation digital health system: increasing effectiveness by optimizing the dosing and minimizing side effects. J Pain Res 15:1051–1060. https://doi.org/10.2147/JPR.S356319
Ilan Y (2020) Second-generation digital health platforms: placing the patient at the center and focusing on clinically meaningful endpoints title: second-generation artificial intelligence algorithms. Front Dig Health. https://doi.org/10.3389/fdgth.2020.569178
Acknowledgements
NA.
Funding
NA.
Author information
Authors and Affiliations
Contributions
YI wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
YI is the founder of Oberon Sciences.
Ethical approval
NA.
Consent to participate
NA.
Consent for publication
The author provides consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ilan, Y. Microtubules as a potential platform for energy transfer in biological systems: a target for implementing individualized, dynamic variability patterns to improve organ function. Mol Cell Biochem 478, 375–392 (2023). https://doi.org/10.1007/s11010-022-04513-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04513-1