Abstract
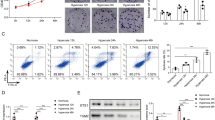
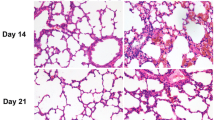
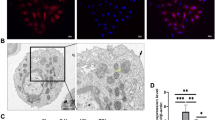
P311 is associated with alveolar formation and development. However, the role and possible mechanism of P311 in hyperoxia-induced injury in type II alveolar epithelial cells (AEC II) need to be elucidated. In our study, rat AEC II (RLE-6TN) were exposure to normoxia (21% O2 and 5% CO2) or hyperoxia (95% O2 and 5% CO2) for 24 h, followed by determination of P311 expression. After knockdown of P311 and hyperoxic treatment, cell viability, cell cycle progression, apoptosis and the Smad3 signaling pathway were examined. Rat AEC II were pretreated with SIS3 HCl for 4 h and then subjected to P311 overexpression plasmid transfection and hyperoxic exposure. Then, cell viability, apoptosis and the Smad3 signaling pathway were determined. The results showed that hyperoxic exposure significantly elevated P311 levels in rat AEC II. P311 knockdown increased cell viability, accelerated cell cycle progression and inhibited apoptosis, as well as suppression of the Smad3 signaling pathway in hyperoxia-exposed AEC II. Additionally, we found that P311 overexpression enhanced the effects of hyperoxia. Interestingly, SIS3 HCl incubation blocked the effects of P311 overexpression on rat AEC II function under hyperoxic condition, as evidenced by an increase in cell viability, and suppressions of apoptosis and the Smad3 signaling pathway. These results indicate that P311 knockdown may ameliorate hyperoxia-induced injury by inhibiting the Smad3 signaling pathway in rat AEC II. P311 may be a novel target for the treatment of hyperoxia-induced lung injury and even bronchopulmonary dysplasia (BPD).





Similar content being viewed by others
References
Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B (2004) Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther 310:512–519. https://doi.org/10.1124/jpet.103.059766
Couroucli XI, Welty SE, Geske RS, Moorthy B (2002) Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol Pharmacol 61:507–515. https://doi.org/10.1124/mol.61.3.507
Scheeren TWL, Belda FJ, Perel A (2018) The oxygen reserve index (ORI): a new tool to monitor oxygen therapy. J Clin Monit Comput 32:379–389. https://doi.org/10.1007/s10877-017-0049-4
Gore A, Muralidhar M, Espey MG, Degenhardt K, Mantell LL (2010) Hyperoxia sensing: from molecular mechanisms to significance in disease. J Immunotoxicol 7:239–254. https://doi.org/10.3109/1547691X.2010.492254
Boggaram V (2004) Regulation of surfactant protein gene expression by hyperoxia in the lung. Antioxid Redox Signal 6:185–190. https://doi.org/10.1089/152308604771978499
Zhang PX, Han CH, Zhou FJ, Li L, Zhang HM, Liu WW (2016) Renin-angiotensin system and its role in hyperoxic acute lung injury. Undersea Hyperb Med 43:239–246
Wang J, Dong W (2018) Oxidative stress and bronchopulmonary dysplasia. Gene 678:177–183. https://doi.org/10.1016/j.gene.2018.08.031
Chen JH, Feng DD, Chen YF, Yang CX, Juan CX, Cao Q, Chen X, Liu S, Zhou GP (2020) Long non-coding RNA MALAT1 targeting STING transcription promotes bronchopulmonary dysplasia through regulation of CREB. J Cell Mol Med 24:10478–10492. https://doi.org/10.1111/jcmm.15661
Studler JM, Glowinski J, Levi-Strauss M (1993) An abundant mRNA of the embryonic brain persists at a high level in cerebellum, hippocampus and olfactory bulb during adulthood. Eur J Neurosci 5:614–623. https://doi.org/10.1111/j.1460-9568.1993.tb00527.x
Duan FF, Barron G, Meliton A, Mutlu GM, Dulin NO, Schuger L (2019) P311 promotes lung fibrosis via stimulation of transforming growth factor-beta1, -beta2, and -beta3 translation. Am J Respir Cell Mol Biol 60:221–231. https://doi.org/10.1165/rcmb.2018-0028OC
Mariani L, McDonough WS, Hoelzinger DB, Beaudry C, Kaczmarek E, Coons SW, Giese A, Moghaddam M, Seiler RW, Berens ME (2001) Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res 61:4190–4196
Tan J, Peng X, Luo G, Ma B, Cao C, He W, Yuan S, Li S, Wilkins JA, Wu J (2010) Investigating the role of P311 in the hypertrophic scar. PLoS ONE 5:e9995. https://doi.org/10.1371/journal.pone.0009995
Zhao L, Leung JK, Yamamoto H, Goswami S, Kheradmand F, Vu TH (2006) Identification of P311 as a potential gene regulating alveolar generation. Am J Respir Cell Mol Biol 35:48–54. https://doi.org/10.1165/rcmb.2005-0475OC
Liu Y, Zhou X, Hu N, Wang C, Zhao L (2020) P311 regulates distal lung development via its interaction with several binding proteins. Mech Dev 163:103633. https://doi.org/10.1016/j.mod.2020.103633
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, Zhao J, Zhang HT (2014) JAK/STAT3 signaling is required for TGF-beta-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol 44:1643–1651. https://doi.org/10.3892/ijo.2014.2310
Pan YQ, Hou AN (2018) Hyperoxia-induced lung injury increases CDKN1A levels in a newborn rat model of bronchopulmonary dysplasia. Exp Lung Res 44:424–432. https://doi.org/10.1080/01902148.2018.1479898
Alam MA, Betal SGN, Aghai ZH, Bhandari V (2019) Hyperoxia causes miR199a-5p-mediated injury in the developing lung. Pediatr Res 86:579–588. https://doi.org/10.1038/s41390-019-0524-3
Zou DM, Zhou SM, Li LH, Zhou JL, Tang ZM, Wang SH (2020) Knockdown of long noncoding RNAs of maternally expressed 3 alleviates hyperoxia-induced lung injury via inhibiting thioredoxin-interacting protein-mediated pyroptosis by binding to miR-18a. Am J Pathol 190:994–1005. https://doi.org/10.1016/j.ajpath.2019.12.013
Bai YX, Fang F, Jiang JL, Xu F (2017) Extrinsic calcitonin gene-related peptide inhibits hyperoxia-induced alveolar epithelial type II cells apoptosis, oxidative stress, and reactive oxygen species (ROS) production by enhancing notch 1 and homocysteine-induced endoplasmic reticulum protein (HERP) expression. Med Sci Monit 23:5774–5782. https://doi.org/10.12659/msm.904549
Sucre JMS, Jetter CS, Loomans H, Williams J, Plosa EJ, Benjamin JT, Young LR, Kropski JA, Calvi CL, Kook S, Wang P, Gleaves L, Eskaros A, Goetzl L, Blackwell TS, Guttentag SH, Zijlstra A (2018) Successful establishment of primary type II alveolar epithelium with 3D organotypic coculture. Am J Respir Cell Mol Biol 59:158–166. https://doi.org/10.1165/rcmb.2017-0442MA
Liu XX, Yu XR, Jia XH, Wang KX, Yu ZY, Lv CJ (2013) Effect of hyperoxia on the viability and proliferation of the primary type II alveolar epithelial cells. Cell Biochem Biophys 67:1539–1546. https://doi.org/10.1007/s12013-013-9658-9
Liu C, Yang L, Dang H, Fang F, Xu F (2014) Effect of Substance P on type II alveolar epithelial cells exposed to hyperoxia and its regulation of the sonic hedgehog signaling pathway. Mol Med Rep 10:1604–1608. https://doi.org/10.3892/mmr.2014.2330
Deng J, Wang SH, Zheng XM, Tang ZM (2022) Calcitonin Gene-related peptide attenuates hyperoxia-induced oxidative damage in alveolar epithelial type II cells through regulating viability and transdifferentiation. Inflammation. https://doi.org/10.1007/s10753-021-01591-z
Wu D, Liang M, Dang H, Fang F, Xu F, Liu C (2018) Hydrogen protects against hyperoxia-induced apoptosis in type II alveolar epithelial cells via activation of PI3K/Akt/Foxo3a signaling pathway. Biochem Biophys Res Commun 495:1620–1627. https://doi.org/10.1016/j.bbrc.2017.11.193
Lu X, Wang C, Wu D, Zhang C, Xiao C, Xu F (2018) Quantitative proteomics reveals the mechanisms of hydrogen-conferred protection against hyperoxia-induced injury in type II alveolar epithelial cells. Exp Lung Res 44:464–475. https://doi.org/10.1080/01902148.2019.1601296
Lu H, Chen X, Lu Y, Zhu H, Tang W, Wang Q (2019) Effects of C/EBPalpha overexpression on alveolar epithelial type II cell proliferation, apoptosis and surfactant protein-C expression after exposure to hyperoxia. BMC Pulm Med 19:142. https://doi.org/10.1186/s12890-019-0911-x
Li LF, Lee CS, Liu YY, Chang CH, Lin CW, Chiu LC, Kao KC, Chen NH, Yang CT (2015) Activation of Src-dependent Smad3 signaling mediates the neutrophilic inflammation and oxidative stress in hyperoxia-augmented ventilator-induced lung injury. Respir Res 16:112. https://doi.org/10.1186/s12931-015-0275-6
Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK (2009) Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 296:L1031–L1041. https://doi.org/10.1152/ajplung.90392.2008
Rehan VK, Sakurai R, Corral J, Krebs M, Ibe B, Ihida-Stansbury K, Torday JS (2010) Antenatally administered PPAR-gamma agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury. Am J Physiol Lung Cell Mol Physiol 299:L672–L680. https://doi.org/10.1152/ajplung.00240.2010
Vijayachandra K, Lee J, Glick AB (2003) Smad3 regulates senescence and malignant conversion in a mouse multistage skin carcinogenesis model. Cancer Res 63:3447–3452
Guda K, Giardina C, Nambiar P, Cui H, Rosenberg DW (2001) Aberrant transforming growth factor-beta signaling in azoxymethane-induced mouse colon tumors. Mol Carcinog 31:204–213. https://doi.org/10.1002/mc.1055
Zelivianski S, Cooley A, Kall R, Jeruss JS (2010) Cyclin-dependent kinase 4-mediated phosphorylation inhibits Smad3 activity in cyclin D-overexpressing breast cancer cells. Mol Cancer Res 8:1375–1387. https://doi.org/10.1158/1541-7786.MCR-09-0537
Cooley A, Zelivianski S, Jeruss JS (2010) Impact of cyclin E overexpression on Smad3 activity in breast cancer cell lines. Cell Cycle 9:4900–4907. https://doi.org/10.4161/cc.9.24.14158
Zou D, Li J, Fan Q, Zheng X, Deng J, Wang S (2019) Reactive oxygen and nitrogen species induce cell apoptosis via a mitochondria-dependent pathway in hyperoxia lung injury. J Cell Biochem 120:4837–4850. https://doi.org/10.1002/jcb.27382
Lagishetty V, Parthasarathy PT, Phillips O, Fukumoto J, Cho Y, Fukumoto I, Bao H, Cox R Jr, Galam L, Lockey RF, Kolliputi N (2014) Dysregulation of CLOCK gene expression in hyperoxia-induced lung injury. Am J Physiol Cell Physiol 306:C999–C1007. https://doi.org/10.1152/ajpcell.00064.2013
Nagato A, Silva FL, Silva AR, Bezerra FS, Oliveira ML, Bello-Klein A, Cristovao Porto L, Santos Valenca S (2009) Hyperoxia-induced lung injury is dose dependent in Wistar rats. Exp Lung Res 35:713–728. https://doi.org/10.3109/01902140902853184
Funding
This work was supported by National Natural Science Foundation of China under Grant Number 81560256.
Author information
Authors and Affiliations
Contributions
HD designed the study and wrote the manuscript. JJ and HD analyzed the data. WJ, LC, MLQ and HD performed the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, J., Wang, J., Li, C. et al. P311 knockdown alleviates hyperoxia-induced injury by inactivating the Smad3 signaling pathway in type II alveolar epithelial cells. Mol Cell Biochem 478, 277–284 (2023). https://doi.org/10.1007/s11010-022-04500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04500-6