Abstract
Purpose
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that affects newborns who need oxygen therapy, and high-concentration oxygen therapy may cause neonatal morbidity and mortality in newborns. E26 oncogene homologue 1 (ETS1) and transglutaminase 2 (TGM2) have been reported to be associated with lung cell injury. However, the mechanism of ETS1 in regulating BPD is still unclear.
Methods
Hyperoxia-induced A549 cells to simulate hyperoxia-induced alveolar epithelial cell injury. MTT assays and colony formation assays were performed to investigate the proliferation of A549 cells. Flow cytometry was carried out to quantify the apoptosis of A549 cells. The expression levels of ETS1 and TGM2 were quantified by qRT–PCR. The protein expression levels of ETS1, TGM2, β-catenin, c-Jun and MET were measured by western blot. Overexpression of ETS1, overexpression of TGM2, overexpression of ETS1 with downregulation of TGM2 and overexpression of TGM2 with inhibition of Wnt/β-catenin pathway were performed to investigate the role of ETS1, TGM2 and Wnt/β-catenin pathways in hyperoxia-induced alveolar epithelial cell injury.
Results
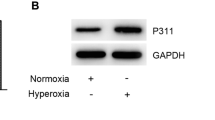
Hyperoxia decreased the proliferation and promoted the apoptosis of cells in a time-dependent manner. Moreover, overexpression of ETS1 rescued the effect of hyperoxia on proliferation and apoptosis. In addition, overexpression of TGM2 participated in the regulation of hyperoxia-induced proliferation and apoptosis. ETS1 regulated hyperoxia-induced alveolar epithelial cell injury through the Wnt/β-catenin pathway via TGM2.
Conclusion
ETS1 ameliorates hyperoxia-induced alveolar epithelial cell injury through the TGM2-mediated Wnt/β-catenin pathway.





Similar content being viewed by others
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- NCPAP:
-
Nasal continuous positive airway pressure
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- EST1 :
-
The E26 oncogene homolog 1
- EBS:
-
ETS binding site
- TGM2 :
-
Transglutaminase 2
References
Haggie S et al (2020) Bronchopulmonary dysplasia: a review of the pulmonary sequelae in the post-surfactant era. J Paediatr Child Health 56(5):680–689
Varghese N, Rios D (2019) Pulmonary hypertension associated with bronchopulmonary dysplasia: a review. Pediatr Allergy Immunol Pulmonol 32(4):140–148
Siffel C et al (2019) Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern-Fetal Neonatal Med 34:1721–1731
Morris IP, Goel N, Chakraborty M (2019) Efficacy and safety of systemic hydrocortisone for the prevention of bronchopulmonary dysplasia in preterm infants: a systematic review and meta-analysis. Eur J Pediatr 178(8):1171–1184
Mosca F, Colnaghi M, Fumagalli M (2011) BPD: old and new problems. J Matern Fetal Neonatal Med 24(Suppl 1):80–82
Gu CC et al (2019) MiR-532–3p suppresses colorectal cancer progression by disrupting the ETS1/TGM2 axis-mediated Wnt/beta-catenin signaling. Cell Death Dis. https://doi.org/10.1038/s41419-019-1962-x
Guo MH et al (2013) The influence of BCSG1 siRNA on gene expression of BCSG1 in human breast cancer and the expression of S-100A4, ETS1, VEGF and CD105 in human breast tumor. Int J Mol Med 32:S74–S74
Kars MD, Iseri OD, Gunduz U (2010) Drug resistant breast cancer cells overexpress ETS1 gene. Biomed Pharmacother 64(7):458–462
Kim GC et al (2020) ETS1 suppresses tumorigenesis of human breast cancer via trans-activation of canonical tumor suppressor genes. Front Oncol. https://doi.org/10.3389/fonc.2020.00642
Yang M et al (2017) Angiogenesis-related genes may be a more important factor than matrix metalloproteinases in bronchopulmonary dysplasia development. Oncotarget 8(12):18670–18679
Choi CM et al (2011) Transglutaminase 2 as an independent prognostic marker for survival of patients with non-adenocarcinoma subtype of non-small cell lung cancer. Mol Cancer. https://doi.org/10.1186/1476-4598-10-119
Hadchouel A et al (2008) Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. Plos One 3(9):e3188
Kim JH et al (2012) Effects of transglutaminase 2 inhibition in ventilator-induced lung injury. Respirology 17:65–65
Lee MY et al (2018) Tissue transglutaminase 2 expression is epigenetically regulated in human lung cancer cells and prevents reactive oxygen species-induced apoptosis. Cancer Manag Res 10:2835–2848
Witsch TJ et al (2014) Transglutaminase 2: a new player in bronchopulmonary dysplasia? Eur Respir J 44(1):109–121
Wang SH et al (2018) The Wnt7b/beta-catenin signaling pathway is involved in the protective action of calcitonin gene-related peptide on hyperoxia-induced lung injury in premature rats. Cell Mol Biol Lett. https://doi.org/10.1186/s11658-018-0071-7
Syed M et al (2017) Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat Commun 8(1):1173
Abdul-Hafez A, Mohamed T, Uhal BD (2019) Activation of mas restores hyperoxia-induced loss of lung epithelial barrier function through inhibition of apoptosis. J Lung Pulm Respir Res 6(3):58–62
Tiwari KK et al (2014) Differential concentration-specific effects of caffeine on cell viability, oxidative stress, and cell cycle in pulmonary oxygen toxicity in vitro. Biochem Biophys Res Commun 450(4):1345–1350
Jia X et al (2021) YAP and Wnt3a independently promote AECIIs proliferation and differentiation by increasing nuclear betacatenin expression in experimental bronchopulmonary dysplasia. Int J Mol Med 47(1):195–206
Yang P et al (2019) TGM2 interference regulates the angiogenesis and apoptosis of colorectal cancer via Wnt/beta-catenin pathway. Cell Cycle 18(10):1122–1134
Chen G, Sun X, Dong C (2017) RhoA regulates lipopolysaccharideinduced lung cell injury via the Wnt/betacatenin pathway. Mol Med Rep 16(6):8501–8506
Cheng L et al (2018) Wnt/beta-catenin pathway promotes acute lung injury induced by LPS through driving the Th17 response in mice. Biochem Biophys Res Commun 495(2):1890–1895
Dasgupta C et al (2009) Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 296(6):L1031–L1041
Funding
This work was supported by Youth Fund of Hunan Natural Science Foundation (No. 2019JJ50293) and Hunan Health Commission (No. B2019012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
408_2021_489_MOESM1_ESM.pdf
Supplementary file1 Schematic diagram: Hyperoxia-induced down-regulation of ETS1 affects the TGM2-mediated Wnt/β-catenin pathway, thereby promoting the apoptosis of alveolar epithelial cell injury and inhibiting proliferation (PDF 155 kb)
Rights and permissions
About this article
Cite this article
Yang, M., Gao, XR., Meng, YN. et al. ETS1 Ameliorates Hyperoxia-Induced Alveolar Epithelial Cell Injury by Regulating the TGM2-Mediated Wnt/β-Catenin Pathway. Lung 199, 681–690 (2021). https://doi.org/10.1007/s00408-021-00489-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-021-00489-9