Abstract
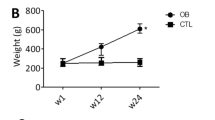
Diet-induced metabolic diseases, such as obesity, metabolic syndrome, and type 2 diabetes (T2DM) are the global threatening epidemics that share cardiovascular oxidative stress as common denominator. Monoamine oxidase (MAO) has recently emerged as a constant source of reactive oxygen species (ROS) in DM. Metformin, the first-line drug in T2DM, elicits cardiovascular protection via pleiotropic effects. The present study was aimed to assess the contribution of MAO to the early cardiac oxidative stress in a rat model of high-calorie junk food (HCJF) diet-induced obesity and prediabetes and whether metformin can alleviate it. After 6 months of HCJF, rats developed obesity and hyperglycemia. Hearts were isolated and used for the evaluation of MAO expression and ROS production. Experiments were performed in the presence vs absence of metformin (10 µM) and MAO-A and B inhibitors (clorgyline and selegiline, 10 µM), respectively. Both MAO isoforms were overexpressed and led to increased ROS generation in cardiac samples harvested from the obese animals. Acute treatment with metformin and MAO inhibitors was able to mitigate oxidative stress. More important, metformin downregulated MAO expression in the diseased samples. In conclusion, MAO contributes to oxidative stress in experimental obesity and can be targeted with metformin.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Heydemann A (2016) An overview of murine high fat diet as a model for type 2 diabetes mellitus. J Diabetes Res 2016:2902351. https://doi.org/10.1155/2016/2902351
Wali JA, Jarzebska N, Raubenheimer D, Simpson SJ, Rodionov RN, O’Sullivan JF (2020) Cardio-metabolic effects of high-fat diets and their underlying mechanisms—a narrative review. Nutrients. https://doi.org/10.3390/nu12051505
Wilson LF, Baade PD, Green AC, Jordan SJ, Kendall BJ, Neale RE, Olsen CM, Youlden DR, Webb PM, Whiteman DC (2019) The impact of changing the prevalence of overweight/obesity and physical inactivity in Australia: an estimate of the proportion of potentially avoidable cancers 2013–2037. Int J Cancer 144:2088–2098. https://doi.org/10.1002/ijc.31943
Izzo C, Vitillo P, Di Pietro P, Visco V, Strianese A, Virtuoso N, Ciccarelli M, Galasso G, Carrizzo A, Vecchione C (2021) The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life (Basel). https://doi.org/10.3390/life11010060
Lau ES, Paniagua SM, Zarbafian S, Hoffman U, Long MT, Hwang SJ, Courchesne P, Yao C, Ma J, Larson MG, Levy D, Shah RV, Ho JE (2021) Cardiovascular biomarkers of obesity and overlap with cardiometabolic dysfunction. J Am Heart Assoc 10:e020215. https://doi.org/10.1161/jaha.120.020215
Wu H, Ballantyne CM (2020) Metabolic inflammation and insulin resistance in obesity. Circ Res 126:1549–1564. https://doi.org/10.1161/circresaha.119.315896
Tipton KF (2018) 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna) 125:1519–1551. https://doi.org/10.1007/s00702-018-1881-5
Youdim MB, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309. https://doi.org/10.1038/nrn1883
Schwartz TL (2013) A neuroscientific update on monoamine oxidase and its inhibitors. CNS Spectr 18 Suppl 1:25–32; quiz 33. https://doi.org/10.1017/s1092852913000734
Tripathi RKP, Ayyannan SR (2019) Monoamine oxidase-B inhibitors as potential neurotherapeutic agents: an overview and update. Med Res Rev 39:1603–1706. https://doi.org/10.1002/med.21561
Sturza A, Leisegang MS, Babelova A, Schröder K, Benkhoff S, Loot AE, Fleming I, Schulz R, Muntean DM, Brandes RP (2013) Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension 62:140–146. https://doi.org/10.1161/hypertensionaha.113.01314
Sturza A, Duicu OM, Vaduva A, Dănilă MD, Noveanu L, Varró A, Muntean DM (2015) Monoamine oxidases are novel sources of cardiovascular oxidative stress in experimental diabetes. Can J Physiol Pharmacol 93:555–561. https://doi.org/10.1139/cjpp-2014-0544
Ionică LN, Gaiță L, Bînă AM, Soşdean R, Lighezan R, Sima A, Malița D, Crețu OM, Burlacu O, Muntean DM, Sturza A (2021) Metformin alleviates monoamine oxidase-related vascular oxidative stress and endothelial dysfunction in rats with diet-induced obesity. Mol Cell Biochem. https://doi.org/10.1007/s11010-021-04194-2
Lighezan R, Sturza A, Duicu OM, Ceausu RA, Vaduva A, Gaspar M, Feier H, Vaida M, Ivan V, Lighezan D, Muntean DM, Mornos C (2016) Monoamine oxidase inhibition improves vascular function in mammary arteries from nondiabetic and diabetic patients with coronary heart disease. Can J Physiol Pharmacol 94:1040–1047. https://doi.org/10.1139/cjpp-2015-0580
Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, Paolocci N (2010) Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106:193–202. https://doi.org/10.1161/circresaha.109.198366
Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, Paolocci N (2014) Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal 20:267–280. https://doi.org/10.1089/ars.2012.4616
Deshwal S, Forkink M, Hu CH, Buonincontri G, Antonucci S, Di Sante M, Murphy MP, Paolocci N, Mochly-Rosen D, Krieg T, Di Lisa F, Kaludercic N (2018) Monoamine oxidase-dependent endoplasmic reticulum-mitochondria dysfunction and mast cell degranulation lead to adverse cardiac remodeling in diabetes. Cell Death Differ 25:1671–1685. https://doi.org/10.1038/s41418-018-0071-1
Manni ME, Rigacci S, Borchi E, Bargelli V, Miceli C, Giordano C, Raimondi L, Nediani C (2016) Monoamine oxidase is overactivated in left and right ventricles from ischemic hearts: an intriguing therapeutic target. Oxid Med Cell Longev 2016:4375418. https://doi.org/10.1155/2016/4375418
Duicu OM, Lighezan R, Sturza A, Balica R, Vaduva A, Feier H, Gaspar M, Ionac A, Noveanu L, Borza C, Muntean DM, Mornos C (2016) Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxid Med Cell Longev 2016:8470394. https://doi.org/10.1155/2016/8470394
Ren J, Wu NN, Wang S, Sowers JR, Zhang Y (2021) Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev 101:1745–1807. https://doi.org/10.1152/physrev.00030.2020
Forouzandeh F, Salazar G, Patrushev N, Xiong S, Hilenski L, Fei B, Alexander RW (2014) Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. J Am Heart Assoc 3:e001202. https://doi.org/10.1161/jaha.114.001202
(2021) 3. prevention or delay of type 2 diabetes: standards of medical care in diabetes-2021. Diabetes Care 44:S34-s39. https://doi.org/10.2337/dc21-S003
Tulipano G (2021) Integrated or independent actions of metformin in target tissues underlying its current use and new possible applications in the endocrine and metabolic disorder area. Int J Mol Sci. https://doi.org/10.3390/ijms222313068
Yerevanian A, Soukas AA (2019) Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep 8:156–164. https://doi.org/10.1007/s13679-019-00335-3
Sturza A, Duicu OM, Vaduva A, Danila MD, Noveanu L, Varro A, Muntean DM (2015) Monoamine oxidases are novel sources of cardiovascular oxidative stress in experimental diabetes. Can J Physiol Pharmacol 93:555–561. https://doi.org/10.1139/cjpp-2014-0544
Sturza A, Muntean DM, Crețu OM (2021) Monoamine oxidase, obesity and related comorbidities: discovering bonds. In: Tappia PS, Ramjiawan B, Dhalla NS (eds) Cellular and biochemical mechanisms of obesity. Springer International Publishing, Cham, pp 199–213
Sorato E, Menazza S, Zulian A, Sabatelli P, Gualandi F, Merlini L, Bonaldo P, Canton M, Bernardi P, Di Lisa F (2014) Monoamine oxidase inhibition prevents mitochondrial dysfunction and apoptosis in myoblasts from patients with collagen VI myopathies. Free Radic Biol Med 75:40–47. https://doi.org/10.1016/j.freeradbiomed.2014.07.006
Mialet-Perez J, Parini A (2020) Cardiac monoamine oxidases: at the heart of mitochondrial dysfunction. Cell Death Dis 11:54. https://doi.org/10.1038/s41419-020-2251-4
Chen L, Guo L, Sun Z, Yang G, Guo J, Chen K, Xiao R, Yang X, Sheng L (2020) Monoamine Oxidase A is a Major Mediator of Mitochondrial Homeostasis and Glycolysis in Gastric Cancer Progression. Cancer Manag Res 12:8023–8035. https://doi.org/10.2147/cmar.s257848
Umbarkar P, Singh S, Arkat S, Bodhankar SL, Lohidasan S, Sitasawad SL (2015) Monoamine oxidase-A is an important source of oxidative stress and promotes cardiac dysfunction, apoptosis, and fibrosis in diabetic cardiomyopathy. Free Radic Biol Med 87:263–273. https://doi.org/10.1016/j.freeradbiomed.2015.06.025
Costiniti V, Spera I, Menabò R, Palmieri EM, Menga A, Scarcia P, Porcelli V, Gissi R, Castegna A, Canton M (2018) Monoamine oxidase-dependent histamine catabolism accounts for post-ischemic cardiac redox imbalance and injury. Biochim Biophys Acta Mol Basis Dis 1864:3050–3059. https://doi.org/10.1016/j.bbadis.2018.06.018
Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A (2005) Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112:3297–3305. https://doi.org/10.1161/circulationaha.104.528133
Kunduzova OR, Bianchi P, Parini A, Cambon C (2002) Hydrogen peroxide production by monoamine oxidase during ischemia/reperfusion. Eur J Pharmacol 448:225–230. https://doi.org/10.1016/s0014-2999(02)01913-1
Inagaki T, Akiyama T, Du CK, Zhan DY, Yoshimoto M, Shirai M (2016) Monoamine oxidase-induced hydroxyl radical production and cardiomyocyte injury during myocardial ischemia-reperfusion in rats. Free Radic Res 50:645–653. https://doi.org/10.3109/10715762.2016.1162300
Deshwal S, Di Sante M, Di Lisa F, Kaludercic N (2017) Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr Opin Pharmacol 33:64–69. https://doi.org/10.1016/j.coph.2017.04.003
Shao W, Shu S, Liu R, Jiang Y, Zhang W, Men H (2019) Monoamine oxidase inhibitors protect against coronary heart disease in rodent rat models: a pilot study. Pak J Pharm Sci 32:371–375
Pino R, Failli P, Mazzetti L, Buffoni F (1997) Monoamine oxidase and semicarbazide-sensitive amine oxidase activities in isolated cardiomyocytes of spontaneously hypertensive rats. Biochem Mol Med 62:188–196. https://doi.org/10.1006/bmme.1997.2633
Mialet-Perez J, Bianchi P, Kunduzova O, Parini A (2007) New insights on receptor-dependent and monoamine oxidase-dependent effects of serotonin in the heart. J Neural Transm (Vienna) 114:823–827. https://doi.org/10.1007/s00702-007-0695-7
Santin Y, Sicard P, Vigneron F, Guilbeau-Frugier C, Dutaur M, Lairez O, Couderc B, Manni D, Korolchuk VI, Lezoualc’h F, Parini A, Mialet-Perez J (2016) Oxidative stress by monoamine oxidase-A impairs transcription factor EB activation and autophagosome clearance, leading to cardiomyocyte necrosis and heart failure. Antioxid Redox Signal 25:10–27. https://doi.org/10.1089/ars.2015.6522
Mialet-Perez J, Santin Y, Parini A (2018) Monoamine oxidase-A, serotonin and norepinephrine: synergistic players in cardiac physiology and pathology. J Neural Transm (Vienna) 125:1627–1634. https://doi.org/10.1007/s00702-018-1908-y
Manni ME, Zazzeri M, Musilli C, Bigagli E, Lodovici M, Raimondi L (2013) Exposure of cardiomyocytes to angiotensin II induces over-activation of monoamine oxidase type A: implications in heart failure. Eur J Pharmacol 718:271–276. https://doi.org/10.1016/j.ejphar.2013.08.022
Kaludercic N, Mialet-Perez J, Paolocci N, Parini A, Di Lisa F (2014) Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol 73:34–42. https://doi.org/10.1016/j.yjmcc.2013.12.032
Kaludercic N, Carpi A, Menabò R, Di Lisa F, Paolocci N (2011) Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta 1813:1323–1332. https://doi.org/10.1016/j.bbamcr.2010.09.010
Deftereos SN, Dodou E, Andronis C, Persidis A (2012) From depression to neurodegeneration and heart failure: re-examining the potential of MAO inhibitors. Expert Rev Clin Pharmacol 5:413–425. https://doi.org/10.1586/ecp.12.29
Corbineau S, Breton M, Mialet-Perez J, Costemale-Lacoste JF (2017) Major depression and heart failure: interest of monoamine oxidase inhibitors. Int J Cardiol 247:1–6. https://doi.org/10.1016/j.ijcard.2017.07.005
Sturza A, Noveanu L, Duicu O, Muntean D (2014) P172Monoamine oxidase inhibition corrects endothelial dysfunction in experimental diabetes. Cardiovasc Res 103:S30–S30. https://doi.org/10.1093/cvr/cvu082.108
Jingying Qiu CL, Dong Z, Wang J (2020) Anti-diabetic effect of a monoamine oxidase inhibitor (tranylcypromine) in rats with poorly-controlled blood glucose levels: a potential and novel therapeutic option for diabetes. Trop J Pharm Res 19:1249–1254. https://doi.org/10.4314/tjpr.v19i6.20
Emory H, Mizrahi N (2017) Glycaemic control by monoamine oxidase inhibition in a patient with type 1 diabetes. Diab Vasc Dis Res 14:163–165. https://doi.org/10.1177/1479164116675492
Sturza A, Popoiu CM, Ionică M, Duicu OM, Olariu S, Muntean DM, Boia ES (2019) Monoamine oxidase-related vascular oxidative stress in diseases associated with inflammatory burden. Oxid Med Cell Longev 2019:8954201. https://doi.org/10.1155/2019/8954201
Battineni G, Sagaro GG, Chintalapudi N, Amenta F, Tomassoni D, Tayebati SK (2021) Impact of obesity-induced inflammation on cardiovascular diseases (CVD). Int J Mol Sci. https://doi.org/10.3390/ijms22094798
Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Wong AK, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G (2016) Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res 119:652–665. https://doi.org/10.1161/circresaha.116.308445
Acknowledgements
This research was funded by the university internal grant code 6POSTDOC/1871/12.02.2020 (A.S.).
Funding
Funding was provided by “Victor Babes” University of Medicine and Pharmacy, Timişoara, Romania (6POSTDOC/1871/12.02.2020).
Author information
Authors and Affiliations
Contributions
APM: investigation, formal analysis LNI: investigation, original draft preparation, formal analysis; AMB: methodology, investigation; SP: visualization, supervision; RL: investigation visualization; LP: supervision; CB: visualization, supervision; A.S.: data curation, writing—review and editing; DMM: conceptualization, writing—review and editing; OMC: project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Merce, A.P., Ionică, L.N., Bînă, A.M. et al. Monoamine oxidase is a source of cardiac oxidative stress in obese rats: the beneficial role of metformin. Mol Cell Biochem 478, 59–67 (2023). https://doi.org/10.1007/s11010-022-04490-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04490-5