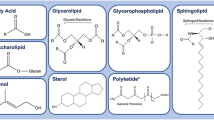
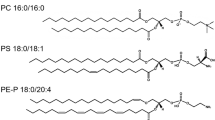
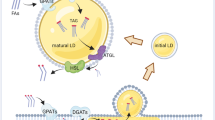
Abstract
Triple-negative breast cancer (TNBC) is a highly aggressive form of breast cancer associated with poor prognosis, higher grade, and a high rate of metastatic occurrence. Limited therapeutic interventions and the compounding issue of drug resistance in triple-negative breast cancer warrants the discovery of novel therapeutic targets and diagnostic modules. To this view, in addition to proteins, lipids also regulate cellular functions via the formation of membranes that modulate membrane protein function, diffusion, and their localization; thus, orchestrating signaling hot spots enriched in specific lipids/proteins on cell membranes. Lipid deregulation in cancer leads to reprogramming of the membrane dynamics and functions impacting cell proliferation, metabolism, and metastasis, providing exciting starting points for developing lipid-based approaches for treating TNBC. In this review, we provide a detailed account of specific lipidic changes in breast cancer, link the altered lipidome with membrane structure and mechanical properties, and describe how these are linked to subsequent downstream functions implicit in cancer progression, metastasis, and chemoresistance. At the fundamental level, we discuss how the lipid-centric findings in TNBC are providing cues for developing lipid-inspired theranostic strategies while bridging existing gaps in our understanding of the functional involvement of lipid membranes in cancer.



Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Yang NJ, Hinner MJ (2015) Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Methods Mol Biol 1266:29–53
Escribá PV, González-Ros JM, Goñi FM et al (2008) Membranes: a meeting point for lipids, proteins and therapies. J Cell Mol Med. https://doi.org/10.1111/j.1582-4934.2008.00281.x
Ibarguren M, López DJ, Encinar JA et al (2013) Partitioning of liquid-ordered/liquid-disordered membrane microdomains induced by the fluidifying effect of 2-hydroxylated fatty acid derivatives. Biochim Biophys Acta-Biomembr 1828:2553–2563. https://doi.org/10.1016/j.bbamem.2013.06.014
Casares D, Escribá PV, Rosselló CA (2019) Membrane lipid composition: effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int J Mol Sci. https://doi.org/10.3390/ijms20092167
Zalba S, ten Hagen TLM (2017) Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat Rev 52:48–57. https://doi.org/10.1016/j.ctrv.2016.10.008
Szlasa W, Zendran I, Zalesińska A, et al (2020) Lipid composition of the cancer cell membrane To cite this version: HAL Id: hal-03089978 Lipid composition of the cancer cell membrane
Preta G (2020) New insights into targeting membrane lipids for cancer therapy. Front Cell Dev Biol 8:1–10. https://doi.org/10.3389/fcell.2020.571237
Kubicek-Sutherland J, Vu D, Mendez H et al (2017) Detection of lipid and amphiphilic biomarkers for disease diagnostics. Biosensors. https://doi.org/10.3390/bios7030025
Escribá PV, Busquets X, Inokuchi JI et al (2015) Membrane lipid therapy: modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res 59:38–53. https://doi.org/10.1016/j.plipres.2015.04.003
Escribá PV (2017) Membrane-lipid therapy: a historical perspective of membrane-targeted therapies—from lipid bilayer structure to the pathophysiological regulation of cells. Biochim Biophys Acta-Biomembr 1859:1493–1506. https://doi.org/10.1016/j.bbamem.2017.05.017
Rodrigues C, Milkovic L, Bujak IT et al (2019) Lipid profile and aquaporin expression under oxidative stress in breast cancer cells of different malignancies. Oxid Med Cell Longev. https://doi.org/10.1155/2019/2061830
Bhattarai S, Saini G, Gogineni K, Aneja R (2020) Quadruple-negative breast cancer: novel implications for a new disease. Breast Cancer Res. https://doi.org/10.1186/s13058-020-01369-5
Yin L, Duan J-J, Bian X-W, Yu S (2020) Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. https://doi.org/10.1186/s13058-020-01296-5
Gracià RS, Bezlyepkina N, Knorr RL et al (2010) Effect of cholesterol on the rigidity of saturated and unsaturated membranes: fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 6:1472–1482. https://doi.org/10.1039/b920629a
Codini M, Garcia-Gil M, Albi E (2021) Cholesterol and sphingolipid enriched lipid rafts as therapeutic targets in cancer. Int J Mol Sci. https://doi.org/10.3390/ijms22020726
Vona R, Iessi E, Matarrese P (2021) Role of cholesterol and lipid rafts in cancer signaling: a promising therapeutic opportunity? Front Cell Dev Biol. https://doi.org/10.3389/fcell.2021.622908
Sezgin E, Levental I, Mayor S, Eggeling C (2017) The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. https://doi.org/10.1038/nrm.2017.16
Pike LJ (2003) Lipid rafts: bringing order to chaos. J Lipid Res. https://doi.org/10.1194/jlr.R200021-JLR200
Grecco HE, Schmick M, Bastiaens PIH (2011) Signaling from the living plasma membrane. Cell. https://doi.org/10.1016/j.cell.2011.01.029
Landreh M, Robinson CV (2015) A new window into the molecular physiology of membrane proteins. J Physiol. https://doi.org/10.1113/jphysiol.2014.283150
Mollinedo F, Gajate C (2015) Lipid rafts as major platforms for signaling regulation in cancer. Adv Biol Regul. https://doi.org/10.1016/j.jbior.2014.10.003
Labilloy A, Youker RT, Bruns JR et al (2014) Altered dynamics of a lipid raft associated protein in a kidney model of Fabry disease. Mol Genet Metab. https://doi.org/10.1016/j.ymgme.2013.10.010
Sonnino S, Aureli M, Grassi S et al (2014) Lipid rafts in neurodegeneration and neuroprotection. Mol Neurobiol. https://doi.org/10.1007/s12035-013-8614-4
Badana AK, Chintala M, Gavara MM et al (2018) Lipid rafts disruption induces apoptosis by attenuating expression of LRP6 and survivin in triple negative breast cancer. Biomed Pharmacother 97:359–368. https://doi.org/10.1016/j.biopha.2017.10.045
Quinn PJ (2012) Lipid–lipid interactions in bilayer membranes: Married couples and casual liaisons. Prog Lipid Res. https://doi.org/10.1016/j.plipres.2012.01.001
Seddon JM, Templer RH (1995) Polymorphism of lipid-water systems. Elsevier, Amsterdam
Cullis PR, De Kruijff B (1979) Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta-Rev Biomembr. https://doi.org/10.1016/0304-4157(79)90012-1
Lladó V, López DJ, Ibarguren M et al (2014) Regulation of the cancer cell membrane lipid composition by NaCHOleate: effects on cell signaling and therapeutical relevance in glioma. Biochim Biophys Acta-Biomembr 1838:1619–1627. https://doi.org/10.1016/j.bbamem.2014.01.027
Yang L, Ding L, Huang HW (2003) New phases of phospholipids and implications to the membrane fusion problem †. Biochemistry. https://doi.org/10.1021/bi0344836
Goñi FM (2014) The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim Biophys Acta-Biomembr. https://doi.org/10.1016/j.bbamem.2014.01.006
Escribá PV (2006) Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol Med. https://doi.org/10.1016/j.molmed.2005.11.004
Fuller N, Rand RP (2001) The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys J. https://doi.org/10.1016/S0006-3495(01)75695-0
Chernomordik LV, Kozlov MM (2008) Mechanics of membrane fusion. Nat Struct Mol Biol. https://doi.org/10.1038/nsmb.1455
Piomelli D, Astarita G, Rapaka R (2007) A neuroscientist’s guide to lipidomics. Nat Rev Neurosci. https://doi.org/10.1038/nrn2233
Holthuis JCM, Menon AK (2014) Lipid landscapes and pipelines in membrane homeostasis. Nature. https://doi.org/10.1038/nature13474
Bell RM, Ballas LM, Coleman RA (1981) Lipid topogenesis. J Lipid Res. https://doi.org/10.1016/S0022-2275(20)34952-X
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. https://doi.org/10.1038/nrm2330
Daum G (1985) Lipids of mitochondria. Biochim Biophys Acta-Rev Biomembr. https://doi.org/10.1016/0304-4157(85)90002-4
Koivuniemi A (2017) The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Lett. https://doi.org/10.1002/1873-3468.12754
Messias MCF, Mecatti GC, Priolli DG, De Oliveira CP (2018) Plasmalogen lipids: functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis 17:1–12. https://doi.org/10.1186/s12944-018-0685-9
Han X, Gross RW (1990) Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry. https://doi.org/10.1021/bi00472a032
Lorent JH, Levental KR, Ganesan L et al (2020) Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol. https://doi.org/10.1038/s41589-020-0529-6
Verkleij A, Zwaal RF, Roelofsen B et al (1973) The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta-Biomembr. https://doi.org/10.1016/0005-2736(73)90143-0
Morrot G, Cribier S, Devaux PF et al (1986) Asymmetric lateral mobility of phospholipids in the human erythrocyte membrane. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.83.18.6863
Gupta A, Korte T, Herrmann A, Wohland T (2020) Plasma membrane asymmetry of lipid organization: fluorescence lifetime microscopy and correlation spectroscopy analysis. J Lipid Res. https://doi.org/10.1194/jlr.D119000364
Schachter D, Abbott RE, Cogan U, Flamm M (1983) Lipid fluidity of the individual hemileaflets of human erythrocyte membranes. Ann N Y Acad Sci. https://doi.org/10.1111/j.1749-6632.1983.tb31671.x
Sanchez SA, Tricerri MA, Gratton E (2012) Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1118288109
Parasassi T, De Stasio G, Ravagnan G et al (1991) Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. https://doi.org/10.1016/S0006-3495(91)82041-0
Parasassi T, Ravagnan G, Rusch RM, Gratton E (1993) Modulation and dynamics of phase properties in phospholipid mixtures detected by Laurdan fluorescence. Photochem Photobiol. https://doi.org/10.1111/j.1751-1097.1993.tb02309.x
Parasassi T, Di Stefano M, Loiero M et al (1994) Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys J. https://doi.org/10.1016/S0006-3495(94)80763-5
Parasassi T, Gratton E (1995) Membrane lipid domains and dynamics as detected by Laurdan fluorescence. J Fluoresc. https://doi.org/10.1007/BF00718783
Golfetto O, Hinde E, Gratton E (2015) The Laurdan spectral phasor method to explore membrane micro-heterogeneity and lipid domains in live cells. Methods Mol Biol 1232:273–90
Levi M, Wilson PV, Cooper OJ, Gratton E (1993) Lipid phases in renal brush border membranes revealed by Laurdan fluorescence. Photochem Photobiol. https://doi.org/10.1111/j.1751-1097.1993.tb02312.x
Sezgin E, Gutmann T, Buhl T et al (2015) Adaptive lipid packing and bioactivity in membrane domains. PLoS ONE. https://doi.org/10.1371/journal.pone.0123930
Kreutzberger AJB, Ji M, Aaron J et al (2019) Rhomboid distorts lipids to break the viscosity-imposed speed limit of membrane diffusion. Science (80- ). https://doi.org/10.1126/science.aao0076
Moon S, Yan R, Kenny SJ et al (2017) Spectrally resolved, functional super-resolution microscopy reveals nanoscale compositional heterogeneity in live-cell membranes. J Am Chem Soc. https://doi.org/10.1021/jacs.7b03846
Sameni S, Malacrida L, Tan Z, Digman MA (2018) Alteration in fluidity of cell plasma membrane in huntington disease revealed by spectral phasor analysis. Sci Rep. https://doi.org/10.1038/s41598-018-19160-0
Ammendolia DA, Bement WM, Brumell JH (2021) Plasma membrane integrity: implications for health and disease. BMC Biol. https://doi.org/10.1186/s12915-021-00972-y
Alves AC, Ribeiro D, Nunes C, Reis S (2016) Biophysics in cancer: the relevance of drug-membrane interaction studies. Biochim Biophys Acta-Biomembr 1858:2231–2244. https://doi.org/10.1016/j.bbamem.2016.06.025
Gu R-X, Baoukina S, Tieleman DP (2020) Phase separation in atomistic simulations of model membranes. J Am Chem Soc. https://doi.org/10.1021/jacs.9b11057
Klymchenko AS (2017) Solvatochromic and fluorogenic dyes as environment-sensitive probes: design and biological applications. Acc Chem Res. https://doi.org/10.1021/acs.accounts.6b00517
Steinkühler J, Sezgin E, Urbančič I et al (2019) Mechanical properties of plasma membrane vesicles correlate with lipid order, viscosity and cell density. Commun Biol 2:1–8. https://doi.org/10.1038/s42003-019-0583-3
Kumamoto Y, Harada Y, Takamatsu T, Tanaka H (2018) Label-free molecular imaging and analysis by Raman spectroscopy. ACTA Histochem Cytochem. https://doi.org/10.1267/ahc.18019
Zhanghao K, Liu W, Li M et al (2020) High-dimensional super-resolution imaging reveals heterogeneity and dynamics of subcellular lipid membranes. Nat Commun. https://doi.org/10.1038/s41467-020-19747-0
Heberle FA, Doktorova M, Scott HL et al (2020) Direct label-free imaging of nanodomains in biomimetic and biological membranes by cryogenic electron microscopy. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2002200117
Yamaji-Hasegawa A, Tsujimoto M (2006) Asymmetric distribution of phospholipids in biomembranes. Biol Pharm Bull. https://doi.org/10.1248/bpb.29.1547
Skotland T, Sandvig K (2019) The role of PS 18:0/18:1 in membrane function. Nat Commun. https://doi.org/10.1038/s41467-019-10711-1
Bernardes N, Fialho A (2018) Perturbing the dynamics and organization of cell membrane components: a new paradigm for cancer-targeted therapies. Int J Mol Sci. https://doi.org/10.3390/ijms19123871
Nicolson GL (2014) The fluid—mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40years. Biochim Biophys Acta-Biomembr. https://doi.org/10.1016/j.bbamem.2013.10.019
Nagata S, Suzuki J, Segawa K, Fujii T (2016) Exposure of phosphatidylserine on the cell surface. Cell Death Differ 23:952–961. https://doi.org/10.1038/cdd.2016.7
Vallabhapurapu SD, Blanco VM, Sulaiman MK et al (2015) Variation in human cancer cell external phosphatidylserine is regulated by flippase activity and intracellular calcium. Oncotarget 6:34375–34388. https://doi.org/10.18632/oncotarget.6045
Voll RE, Herrmann M, Roth EA et al (1997) Immunosuppressive effects of apoptotic cells. Nature. https://doi.org/10.1038/37022
Voll RE, Roth EA, Girkontaite I et al (1997) Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. https://doi.org/10.1002/art.1780401210
Cvetanovic M, Ucker DS (2004) Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. https://doi.org/10.4049/jimmunol.172.2.880
Birge RB, Boeltz S, Kumar S et al (2016) Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ 23:962–978. https://doi.org/10.1038/CDD.2016.11
Wanderley JLM, DaMatta RA, Barcinski MA (2020) Apoptotic mimicry as a strategy for the establishment of parasitic infections: parasite- and host-derived phosphatidylserine as key molecule. Cell Commun Signal. https://doi.org/10.1186/s12964-019-0482-8
Bogdanov M, Dowhan W (1998) Phospholipid-assisted protein folding: phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J. https://doi.org/10.1093/emboj/17.18.5255
Patel D, Witt SN (2017) Ethanolamine and phosphatidylethanolamine: partners in health and disease. Oxid Med Cell Longev. https://doi.org/10.1155/2017/4829180
Pustylnikov S, Costabile F, Beghi S, Facciabene A (2018) Targeting mitochondria in cancer: current concepts and immunotherapy approaches. Transl Res. https://doi.org/10.1016/j.trsl.2018.07.013
Beloribi-Djefaflia S, Vasseur S, Guillaumond F (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis. https://doi.org/10.1038/oncsis.2015.49
Krawitz PM, Murakami Y, Rieß A et al (2013) PGAP2 mutations, affecting the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation syndrome. Am J Hum Genet. https://doi.org/10.1016/j.ajhg.2013.03.011
Henriksen JR, Andresen TL, Feldborg LN et al (2010) Understanding detergent effects on lipid membranes: a model study of lysolipids. Biophys J. https://doi.org/10.1016/j.bpj.2010.01.037
Yeagle PL (2016) Biogenesis of Membrane Lipids. In: The Membranes of Cells. Elsevier
Gibellini F, Smith TK (2010) The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. https://doi.org/10.1002/iub.337
Podo F, Paris L, Cecchetti S et al (2016) Activation of phosphatidylcholine-specific phospholipase C in breast and ovarian cancer: impact on MRS-detected choline metabolic profile and perspectives for targeted therapy. Front Oncol. https://doi.org/10.3389/fonc.2016.00171
Abalsamo L, Spadaro F, Bozzuto G et al (2012) Inhibition of phosphatidylcholine-specific phospholipase C results in loss of mesenchymal traits in metastatic breast cancer cells. Breast Cancer Res. https://doi.org/10.1186/bcr3151
Paris L, Podo F, Spadaro F et al (2017) Phosphatidylcholine-specific phospholipase C inhibition reduces HER2-overexpression, cell proliferation and in vivo tumor growth in a highly tumorigenic ovarian cancer model. Oncotarget. https://doi.org/10.18632/oncotarget.18992
Carrasco S, Mérida I (2007) Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. https://doi.org/10.1016/j.tibs.2006.11.004
Rauch C, Paine SW, Littlewood P (2013) Can long range mechanical interaction between drugs and membrane proteins define the notion of molecular promiscuity? Application to P-glycoprotein-mediated multidrug resistance (MDR). Biochim Biophys Acta-Gen Subj. https://doi.org/10.1016/j.bbagen.2013.06.038
Rivel T, Ramseyer C, Yesylevskyy S (2019) The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-41903-w
Peetla C, Bhave R, Vijayaraghavalu S et al (2010) Drug resistance in breast cancer cells: biophysical characterization of and doxorubicin interactions with membrane lipids. Mol Pharm 7:2334–2348. https://doi.org/10.1021/mp100308n
Munir R, Lisec J, Swinnen JV, Zaidi N (2019) Lipid metabolism in cancer cells under metabolic stress. Br J Cancer. https://doi.org/10.1038/s41416-019-0451-4
Snaebjornsson MT, Janaki-Raman S, Schulze A (2020) Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab 31:62–76. https://doi.org/10.1016/j.cmet.2019.11.010
Zaidi N, Lupien L, Kuemmerle NB et al (2013) Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. https://doi.org/10.1016/j.plipres.2013.08.005
Ferreri C, Sansone A, Ferreri R et al (2020) Fatty acids and membrane lipidomics in oncology: a cross-road of nutritional, signaling and metabolic pathways. Metabolites 10:1–26. https://doi.org/10.3390/metabo10090345
Azordegan N, Fraser V, Le K et al (2013) Carcinogenesis alters fatty acid profile in breast tissue. Mol Cell Biochem 374:223–232. https://doi.org/10.1007/s11010-012-1523-4
Flavin R, Peluso S, Nguyen PL, Loda M (2010) Fatty acid synthase as a potential therapeutic target in cancer. Futur Oncol. https://doi.org/10.2217/fon.10.11
Igal RA (2016) Stearoyl CoA desaturase-1: new insights into a central regulator of cancer metabolism. Biochim Biophys Acta-Mol Cell Biol Lipids. https://doi.org/10.1016/j.bbalip.2016.09.009
Igal RA (2010) Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. https://doi.org/10.1093/carcin/bgq131
Bensaad K, Favaro E, Lewis CA et al (2014) Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. https://doi.org/10.1016/j.celrep.2014.08.056
Samanta S, Sharma VM, Khan A, Mercurio AM (2012) Regulation of IMP3 by EGFR signaling and repression by ERβ: implications for triple-negative breast cancer. Oncogene. https://doi.org/10.1038/onc.2011.620
Schug ZT, Peck B, Jones DT et al (2015) Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. https://doi.org/10.1016/j.ccell.2014.12.002
de Gonzalo-Calvo D, López-Vilaró L, Nasarre L et al (2015) Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. https://doi.org/10.1186/s12885-015-1469-5
Yue S, Li J, Lee S-Y et al (2014) Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. https://doi.org/10.1016/j.cmet.2014.01.019
Vriens K, Christen S, Parik S et al (2019) Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature. https://doi.org/10.1038/s41586-019-0904-1
Scanferlato R, Bortolotti M, Sansone A et al (2019) Hexadecenoic fatty acid positional isomers and De Novo PUFA synthesis in colon cancer cells. Int J Mol Sci. https://doi.org/10.3390/ijms20040832
Leone RD, Amaravadi RK (2013) Autophagy: a targetable linchpin of cancer cell metabolism. Trends Endocrinol Metab 24:209–217. https://doi.org/10.1016/j.tem.2013.01.008
Pandey A, Yadav P, Shukla S (2021) Unfolding the role of autophagy in the cancer metabolism. Biochem Biophys Reports 28:101158. https://doi.org/10.1016/j.bbrep.2021.101158
Maan M, Peters JM, Dutta M, Patterson AD (2018) Lipid metabolism and lipophagy in cancer. Biochem Biophys Res Commun 504:582–589. https://doi.org/10.1016/j.bbrc.2018.02.097
Monaco ME (2017) Fatty acid metabolism in breast cancer subtypes. Oncotarget. https://doi.org/10.18632/oncotarget.15494
Cooke M, Orlando U, Maloberti P et al (2011) Tyrosine phosphatase SHP2 regulates the expression of acyl-CoA synthetase ACSL4. J Lipid Res. https://doi.org/10.1194/jlr.M015552
Zhao H, Agazie YM (2015) Inhibition of SHP2 in basal-like and triple-negative breast cells induces basal-to-luminal transition, hormone dependency, and sensitivity to anti-hormone treatment. BMC Cancer. https://doi.org/10.1186/s12885-015-1131-2
Sausgruber N, Coissieux M-M, Britschgi A et al (2015) Tyrosine phosphatase SHP2 increases cell motility in triple-negative breast cancer through the activation of SRC-family kinases. Oncogene. https://doi.org/10.1038/onc.2014.170
Sun X, Wang M, Wang M et al (2020) Metabolic reprogramming in triple-negative breast cancer. Front Oncol. https://doi.org/10.3389/fonc.2020.00428
Abramczyk H, Surmacki J, Kopeć M et al (2015) The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7, and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst. https://doi.org/10.1039/C4AN01875C
He J, Zhang F, Rachel Tay LW et al (2017) Lipin-1 regulation of phospholipid synthesis maintains endoplasmic reticulum homeostasis and is critical for triple-negative breast cancer cell survival. FASEB J. https://doi.org/10.1096/fj.201601353R
Pucer A, Brglez V, Payré C et al (2013) Group X secreted phospholipase A2 induces lipid droplet formation and prolongs breast cancer cell survival. Mol Cancer. https://doi.org/10.1186/1476-4598-12-111
Han S, Huh J, Kim W et al (2014) Phospholipase D activates HIF-1-VEGF pathway via phosphatidic acid. Exp Mol Med. https://doi.org/10.1038/emm.2014.86
Herman MA, Peroni OD, Villoria J et al (2012) A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484:333–338. https://doi.org/10.1038/nature10986
Lei Y, Zhou S, Hu Q et al (2020) Carbohydrate response element binding protein (ChREBP) correlates with colon cancer progression and contributes to cell proliferation. Sci Rep 10:4233. https://doi.org/10.1038/s41598-020-60903-9
Iizuka K, Bruick RK, Liang G et al (2004) Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci 101:7281–7286. https://doi.org/10.1073/pnas.0401516101
Bindesbøll C, Fan Q, Nørgaard RC et al (2015) Liver X receptor regulates hepatic nuclear O-GlcNAc signaling and carbohydrate responsive element-binding protein activity. J Lipid Res 56:771–785. https://doi.org/10.1194/jlr.M049130
Steffensen K, Jan-Åke K (2006) Liver X receptors: new drug targets to treat Type 2 diabetes? Future Lipidol 1:181–189. https://doi.org/10.2217/17460875.1.2.181
Guo D, Reinitz F, Youssef M et al (2011) An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR–dependent pathway. Cancer Discov 1:442–456. https://doi.org/10.1158/2159-8290.CD-11-0102
Jeong D-W, Lee S, Chun Y-S (2021) How cancer cells remodel lipid metabolism: strategies targeting transcription factors. Lipids Health Dis 20:163. https://doi.org/10.1186/s12944-021-01593-8
Vedin L-L, Lewandowski SA, Parini P et al (2009) The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis 30:575–579. https://doi.org/10.1093/carcin/bgp029
Matsubara T, Li F, Gonzalez FJ (2013) FXR signaling in the enterohepatic system. Mol Cell Endocrinol 368:17–29. https://doi.org/10.1016/j.mce.2012.05.004
Silva J, Dasgupta S, Wang G et al (2006) Lipids isolated from bone induce the migration of human breast cancer cells. J Lipid Res 47:724–733. https://doi.org/10.1194/jlr.M500473-JLR200
Peters JM, Hennuyer N, Staels B et al (1997) Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor α-deficient mice. J Biol Chem 272:27307–27312. https://doi.org/10.1074/jbc.272.43.27307
Her N-H, Jeong S-I, Cho K et al (2013) PPARδ promotes oncogenic redirection of TGF-β1 signaling through the activation of the ABCA1-Cav1 pathway. Cell Cycle 12:1521–1535. https://doi.org/10.4161/cc.24636
Yahagi N, Shimano H, Hasegawa K et al (2005) Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer 41:1316–1322. https://doi.org/10.1016/j.ejca.2004.12.037
Min HK, Kong G, Moon MH (2010) Quantitative analysis of urinary phospholipids found in patients with breast cancer by nanoflow liquid chromatography–tandem mass spectrometry: II Negative ion mode analysis of four phospholipid classes. Anal Bioanal Chem. https://doi.org/10.1007/s00216-009-3292-9
Wang S, Chen X, Luan H et al (2016) Matrix-assisted laser desorption/ionization mass spectrometry imaging of cell cultures for the lipidomic analysis of potential lipid markers in human breast cancer invasion. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.7466
Kawashima M, Iwamoto N, Kawaguchi-Sakita N et al (2013) High-resolution imaging mass spectrometry reveals detailed spatial distribution of phosphatidylinositols in human breast cancer. Cancer Sci. https://doi.org/10.1111/cas.12229
Hosokawa Y, Masaki N, Takei S et al (2017) Recurrent triple-negative breast cancer (TNBC) tissues contain a higher amount of phosphatidylcholine (32:1) than non-recurrent TNBC tissues. PLoS ONE. https://doi.org/10.1371/journal.pone.0183724
Silva CL, Perestrelo R, Sousa-Ferreira I et al (2020) Lipid biosignature of breast cancer tissues by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-020-05672-9
Eiriksson FF, Nøhr MK, Costa M et al (2020) Lipidomic study of cell lines reveals differences between breast cancer subtypes. PLoS ONE 15:1–22. https://doi.org/10.1371/journal.pone.0231289
Kim H-Y, Lee K-M, Kim S-H et al (2016) Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget. https://doi.org/10.18632/oncotarget.11560
Purwaha P, Gu F, Piyarathna D et al (2018) Unbiased lipidomic profiling of triple-negative breast cancer tissues reveals the association of sphingomyelin levels with patient disease-free survival. Metabolites. https://doi.org/10.3390/metabo8030041
Tabatabaei M, Tafazzoli-Shadpour M, Khani MM (2019) Correlation of the cell mechanical behavior and quantified cytoskeletal parameters in normal and cancerous breast cell lines. Biorheology. https://doi.org/10.3233/BIR-190214
Li QS, Lee GYH, Ong CN, Lim CT (2008) AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2008.07.078
Luo Q, Kuang D, Zhang B, Song G (2016) Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim Biophys Acta-Gen Subj 1860:1953–1960. https://doi.org/10.1016/j.bbagen.2016.06.010
Yubero ML, Kosaka PM, San Paulo Á et al (2020) Effects of energy metabolism on the mechanical properties of breast cancer cells. Commun Biol. https://doi.org/10.1038/s42003-020-01330-4
Wirtz D, Konstantopoulos K, Searson P (2012) Mechanical forces in metastasis. Biomol Eng 11:512–522. https://doi.org/10.1038/nrc3080.The
Betapudi V, Licate LS, Egelhoff TT (2006) Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-05-4236
Smelser AM, Macosko JC, O’Dell AP et al (2015) Mechanical properties of normal versus cancerous breast cells. Biomech Model Mechanobiol 14:1335–1347. https://doi.org/10.1007/s10237-015-0677-x
Calzado-Martín A, Encinar M, Tamayo J et al (2016) Effect of actin organization on the stiffness of living breast cancer cells revealed by peak-force modulation atomic force microscopy. ACS Nano. https://doi.org/10.1021/acsnano.5b07162
Smolyakov G, Thiebot B, Campillo C et al (2016) Elasticity, adhesion, and tether extrusion on breast cancer cells provide a signature of their invasive potential. ACS Appl Mater Interfaces 8:27426–27431. https://doi.org/10.1021/acsami.6b07698
Onwudiwe K, Hu J, Obayemi J et al (2021) Actin cytoskeletal structure and the statistical variations of the mechanical properties of non-tumorigenic breast and triple-negative breast cancer cells. J Mech Behav Biomed Mater. https://doi.org/10.1016/j.jmbbm.2021.104505
Hui TH, Zhou ZL, Fong HW et al (2016) Characterizing the malignancy and drug resistance of cancer cells from their membrane resealing response. Sci Rep 6:1–8. https://doi.org/10.1038/srep26692
Bouvet F, Ros M, Bonedeau E et al (2020) Defective membrane repair machinery impairs survival of invasive cancer cells. Sci Rep. https://doi.org/10.1038/s41598-020-77902-5
Boye TL, Nylandsted J (2016) Annexins in plasma membrane repair. Biol Chem. https://doi.org/10.1515/hsz-2016-0171
Jaiswal JK, Nylandsted J (2015) S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle. https://doi.org/10.1080/15384101.2014.995495
Li M, Xi N, Wang Y, Liu L (2021) Atomic force microscopy for revealing micro/nanoscale mechanics in tumor metastasis: from single cells to microenvironmental cues. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-020-0494-3
Ríos-Marco P, Marco C, Gálvez X et al (2017) Alkylphospholipids: an update on molecular mechanisms and clinical relevance. Biochim Biophys Acta-Biomembr 1859:1657–1667. https://doi.org/10.1016/j.bbamem.2017.02.016
Davis HW, Vallabhapurapu SD, Chu Z et al (2019) Enhanced phosphatidylserine-selective cancer therapy with irradiation and SapC-DOPS nanovesicles. Oncotarget. https://doi.org/10.18632/oncotarget.26615
Chang W, Fa H, Xiao D, Wang J (2020) Targeting phosphatidylserine for Cancer therapy: prospects and challenges. Theranostics. https://doi.org/10.7150/thno.45125
Belzile O, Huang X, Gong J et al (2018) Antibody targeting of phosphatidylserine for the detection and immunotherapy of cancer. ImmunoTargets Ther. https://doi.org/10.2147/ITT.S134834
Chalasani P, Marron M, Roe D et al (2015) A phase I clinical trial of bavituximab and paclitaxel in patients with HER2 negative metastatic breast cancer. Cancer Med. https://doi.org/10.1002/cam4.447
Desai TJ, Toombs JE, Minna JD et al (2016) Identification of lipid-phosphatidylserine (PS) as the target of unbiasedly selected cancer specific peptide-peptoid hybrid PPS1. Oncotarget. https://doi.org/10.18632/oncotarget.8929
Desai TJ, Udugamasooriya DG (2017) A comprehensive lipid binding and activity validation of a cancer-specific peptide-peptoid hybrid PPS1. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2017.03.083
De M, Ghosh S, Asad M et al (2020) Combining doxorubicin with stearylamine-bearing liposomes elicits Th1 cytokine responses and cures metastasis in a mouse model. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-020-02578-9
De M, Ghosh S, Sen T et al (2018) A novel therapeutic strategy for cancer using phosphatidylserine targeting stearylamine-bearing cationic liposomes. Mol Ther-Nucleic Acids. https://doi.org/10.1016/j.omtn.2017.10.019
Hullin-Matsuda F, Makino A, Murate M, Kobayashi T (2016) Probing phosphoethanolamine-containing lipids in membranes with duramycin/cinnamycin and aegerolysin proteins. Biochimie 130:81–90. https://doi.org/10.1016/j.biochi.2016.09.020
Iwamoto K, Hayakawa T, Murate M et al (2007) Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophys J 93:1608–1619. https://doi.org/10.1529/biophysj.106.101584
Wang CK, Wacklin HP, Craik DJ (2012) Cyclotides insert into lipid bilayers to form membrane pores and destabilize the membrane through hydrophobic and phosphoethanolamine-specific interactions. J Biol Chem. https://doi.org/10.1074/jbc.M112.421198
Delmas D, Aires V, Colin DJ et al (2013) Importance of lipid microdomains, rafts, in absorption, delivery, and biological effects of resveratrol. Ann N Y Acad Sci. https://doi.org/10.1111/nyas.12177
Gao M, Zhou J, Su Z, Huang Y (2017) Bacterial cupredoxin azurin hijacks cellular signaling networks: protein-protein interactions and cancer therapy. Protein Sci. https://doi.org/10.1002/pro.3310
Huang F, Shu Q, Qin Z et al (2020) Anticancer actions of azurin and its derived peptide p28. Protein J. https://doi.org/10.1007/s10930-020-09891-3
Bernardes N, Garizo AR, Pinto SN et al (2018) Azurin interaction with the lipid raft components ganglioside GM-1 and caveolin-1 increases membrane fluidity and sensitivity to anti-cancer drugs. Cell Cycle. https://doi.org/10.1080/15384101.2018.1489178
Zhang J, Li Q, Wu Y et al (2019) Cholesterol content in cell membrane maintains surface levels of ErbB2 and confers a therapeutic vulnerability in ErbB2-positive breast cancer. Cell Commun Signal. https://doi.org/10.1186/s12964-019-0328-4
Penkauskas T, Zentelyte A, Ganpule S et al (2020) Pleiotropic effects of statins via interaction with the lipid bilayer: a combined approach. Biochim Biophys Acta-Biomembr. https://doi.org/10.1016/j.bbamem.2020.183306
Sahu SS, Sarkar P, Shrivastava S, Chattopadhyay A (2019) Differential effects of simvastatin on membrane organization and dynamics in varying phases. Chem Phys Lipids. https://doi.org/10.1016/j.chemphyslip.2019.104831
Sariisik E, Koçak M, Kucuk Baloglu F, Severcan F (2019) Interaction of the cholesterol reducing agent simvastatin with zwitterionic DPPC and charged DPPG phospholipid membranes. Biochim Biophys Acta-Biomembr. https://doi.org/10.1016/j.bbamem.2019.01.014
Beckwitt CH, Shiraha K, Wells A (2018) Lipophilic statins limit cancer cell growth and survival, via involvement of Akt signaling. PLoS ONE. https://doi.org/10.1371/journal.pone.0197422
Ahern TP, Pedersen L, Tarp M et al (2011) Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. JNCI J Natl Cancer Inst. https://doi.org/10.1093/jnci/djr291
Ahmadi Y, Karimian R, Panahi Y (2018) Effects of statins on the chemoresistance—the antagonistic drug-drug interactions versus the anti-cancer effects. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2018.09.122
Erazo-Oliveras A, Fuentes NR, Wright RC, Chapkin RS (2018) Functional link between plasma membrane spatiotemporal dynamics, cancer biology, and dietary membrane-altering agents. Cancer Metastas Rev 37:519–544. https://doi.org/10.1007/s10555-018-9733-1
Safwat S, Hathout RM, Ishak RA, Mortada ND (2017) Augmented simvastatin cytotoxicity using optimized lipid nanocapsules: a potential for breast cancer treatment. J Liposome Res. https://doi.org/10.3109/08982104.2015.1137313
Matusewicz L, Filip-Psurska B, Psurski M et al (2019) EGFR-targeted immunoliposomes as a selective delivery system of simvastatin, with potential use in treatment of triple-negative breast cancers. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2019.118605
Silva-Cázares M, Saavedra-Leos M, Jordan-Alejandre E et al (2020) Lipid-based nanoparticles for the therapeutic delivery of non-coding RNAs in breast cancer (Review). Oncol Rep. https://doi.org/10.3892/or.2020.7791
Akshaya RL, Akshaya N, Selvamurugan N (2020) A computational study of non-coding RNAs on the regulation of activating transcription factor 3 in human breast cancer cells. Comput Biol Chem. https://doi.org/10.1016/j.compbiolchem.2020.107386
Vaidya AM, Sun Z, Ayat N et al (2019) Systemic delivery of tumor-targeting siRNA nanoparticles against an oncogenic LncRNA facilitates effective triple-negative breast cancer therapy. Bioconjug Chem. https://doi.org/10.1021/acs.bioconjchem.9b00028
Jin S-J, Jin M-Z, Xia B-R, Jin W-L (2019) Long non-coding RNA DANCR as an emerging therapeutic target in human cancers. Front Oncol. https://doi.org/10.3389/fonc.2019.01225
Saraiva SM, Gutiérrez-Lovera C, Martínez-Val J et al (2021) Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model. Sci Rep 11:1–13. https://doi.org/10.1038/s41598-021-87968-4
De Vita A, Liverani C, Molinaro R et al (2021) Lysyl oxidase engineered lipid nanovesicles for the treatment of triple negative breast cancer. Sci Rep. https://doi.org/10.1038/s41598-021-84492-3
Doll S, Proneth B, Tyurina YY et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. https://doi.org/10.1038/nchembio.2239
Li J, Cao F, Yin H et al (2020) Ferroptosis: past, present and future. Cell Death Dis. https://doi.org/10.1038/s41419-020-2298-2
Yang WS, SriRamaratnam R, Welsch ME et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell. https://doi.org/10.1016/j.cell.2013.12.010
Yu H, Yang C, Jian L et al (2019) Sulfasalazine-induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol Rep. https://doi.org/10.3892/or.2019.7189
Hangauer MJ, Viswanathan VS, Ryan MJ et al (2017) Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. https://doi.org/10.1038/nature24297
Chen M-S, Wang S-F, Hsu C-Y et al (2017) CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget. https://doi.org/10.18632/oncotarget.23055
Guerra ÂR, Paulino AF, Castro MM et al (2020) Triple negative breast cancer and breast epithelial cells differentially reprogram glucose and lipid metabolism upon treatment with triterpenic acids. Biomolecules 10:1–18. https://doi.org/10.3390/biom10081163
Wright HJ, Hou J, Xu B et al (2017) CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1703791114
Guardiola-Serrano F, Beteta-Göbel R, Rodríguez-Lorca R et al (2019) The triacylglycerol, hydroxytriolein, inhibits triple negative mammary breast cancer cell proliferation through a mechanism dependent on dihydroceramide and Akt. Oncotarget 10:2486–2507. https://doi.org/10.18632/oncotarget.26824
Bulbake U, Doppalapudi S, Kommineni N, Khan W (2017) Liposomal formulations in clinical use: an updated review. Pharmaceutics 9:12. https://doi.org/10.3390/pharmaceutics9020012
Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I et al (2019) Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol 10:11. https://doi.org/10.1186/s12645-019-0055-y
Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. Int J Nanomed. https://doi.org/10.2147/IJN.S68861
Miller K, Cortes J, Hurvitz SA et al (2016) HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 16:352. https://doi.org/10.1186/s12885-016-2385-z
Harbeck N, Saupe S, Jäger E et al (2017) A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine as first-line therapy for metastatic breast cancer: results of the PELICAN study. Breast Cancer Res Treat 161:63–72. https://doi.org/10.1007/s10549-016-4033-3
Jehn CF, Hemmati P, Lehenbauer-Dehm S et al (2016) Biweekly pegylated liposomal doxorubicin (Caelyx) in heavily pretreated metastatic breast cancer: a phase 2 study. Clin Breast Cancer 16:514–519. https://doi.org/10.1016/j.clbc.2016.06.001
Chang AE, Wu QV, Jenkins IC et al (2018) Phase I/II trial of combined pegylated liposomal doxorubicin and cyclophosphamide in metastatic breast cancer. Clin Breast Cancer 18:e143–e149. https://doi.org/10.1016/j.clbc.2017.10.005
Basho RK, Gilcrease M, Murthy RK et al (2017) Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer. JAMA Oncol 3:509. https://doi.org/10.1001/jamaoncol.2016.5281
Awada A, Bondarenko IN, Bonneterre J et al (2014) A randomized controlled phase II trial of a novel composition of paclitaxel embedded into neutral and cationic lipids targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann Oncol 25:824–831. https://doi.org/10.1093/annonc/mdu025
Ahn HK, Jung M, Sym SJ et al (2014) A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol 74:277–282. https://doi.org/10.1007/s00280-014-2498-5
Acknowledgements
We acknowledge IIT Bombay for all central facilities.
Funding
Work in the SK lab is supported by the DST-SERB Grant EMR/2016/005414 and WEA/2020/000032, BRNS Grant 201805BRE01RP04922, Wadhwani Research Centre for Bioengineering (WRCB), DBT Ramalingaswami Fellowship, and the DST-Inspire Faculty Award. RD acknowledges funding from DST Women Scientist Scheme WOS-A/CS-16/2018.
Author information
Authors and Affiliations
Contributions
SK and RD conceived the idea, wrote the review, and made the figures. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dadhich, R., Kapoor, S. Lipidomic and Membrane Mechanical Signatures in Triple-Negative Breast Cancer: Scope for Membrane-Based Theranostics. Mol Cell Biochem 477, 2507–2528 (2022). https://doi.org/10.1007/s11010-022-04459-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04459-4