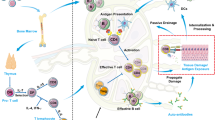
Abstract
Autoimmune diseases are caused by the immune response of the body to its antigens, resulting in tissue damage. The pathogenesis of these diseases has not yet been elucidated. Most autoimmune diseases cannot be cured by effective drugs. The treatment strategy is to relieve the symptoms of the disease and balance the body’s autoimmune function. The abnormal expression of ATP-binding cassette (ABC) transporters is directly related to the pathogenesis of autoimmune diseases and drug therapy resistance, which poses a great challenge for the drug therapy of autoimmune diseases. Therefore, this paper reviews the interplay between ABC transporters and the pathogenesis of autoimmune diseases to provide research progress and new ideas for the development of drugs in autoimmune diseases.







Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- ABC:
-
ATP-binding cassette
- ATP:
-
Adenosine triphosphate
- NBD:
-
Nucleotide-binding domain
- TMD:
-
Transmembrane domain
- ISDs:
-
Immunosuppressive drugs
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- MDR:
-
Multidrug resistance
- TMs:
-
Membrane‑spanning α‑helices
- P-gp:
-
P-glycoprotein
- MRP:
-
Multidrug resistance-associated protein
- BCRP:
-
Breast cancer resistance protein
- MTX:
-
Methotrexate
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-α
- PM:
-
Peritoneal macrophages
- FLSs:
-
Fibroblast-like synoviocytes
- GCs:
-
Glucocorticoids
- PBLs:
-
Peripheral blood lymphocytes
- YB-1:
-
Y-box-binding protein-1
- SSZ:
-
Sulfasalazine
- LEF:
-
Leflunomide
- CP-25:
-
Benzenesulfonyl paeoniflorin
- PBMCs:
-
Peripheral blood mononuclear cells
- DCs:
-
Dendritic cells
- SNP:
-
Single nucleotide polymorphism
- IL-23R:
-
IL-23 receptor
- HLA-B27:
-
Human leukocyte antigen class I
References
Rahman S, McHaourab HS (2020) ATP-dependent interactions of a cargo protein with the transmembrane domain of a polypeptide processing and secretion ABC transporter. J Biol Chem 295:14678–14685. https://doi.org/10.1074/jbc.RA120.014934
Mandal SK, Adhikari R, Sharma A, Chandravanshi M, Gogoi P, Kanaujia SP (2019) Designating ligand specificities to metal uptake ABC transporters in Thermus thermophilus HB8. Metallomics 11:597–612. https://doi.org/10.1039/c8mt00374b
Schmitt L, Tampe R (2002) Structure and mechanism of ABC transporters. Curr Opin Struct Biol 12:754–760. https://doi.org/10.1016/s0959-440x(02)00399-8
Stefan SM (2019) Multi-target ABC transporter modulators: what next and where to go? Future Med Chem 11:2353–2358. https://doi.org/10.4155/fmc-2019-0185
Tucker TG, Milne AM, Fournel-Gigleux S, Fenner KS, Coughtrie MW (2012) Absolute immunoquantification of the expression of ABC transporters P-glycoprotein, breast cancer resistance protein and multidrug resistance-associated protein 2 in human liver and duodenum. Biochem Pharmacol 83:279–285. https://doi.org/10.1016/j.bcp.2011.10.017
van de Water FM, Boleij JM, Peters JGP, Russel FGM, Masereeuw R (2007) Characterization of P-glycoprotein and multidrug resistance proteins in rat kidney and intestinal cell lines. Eur J Pharm Sci 30:36–44. https://doi.org/10.1016/j.ejps.2006.09.008
Yu J, Zhou P, Asenso J, Yang XD, Wang C, Wei W (2016) Advances in plant-based inhibitors of P-glycoprotein. J Enzyme Inhib Med Chem 31:867–881. https://doi.org/10.3109/14756366.2016.1149476
Langan D, Rose NR, Moudgil KD (2020) Common innate pathways to autoimmune disease. Clin Immunol 212:108361. https://doi.org/10.1016/j.clim.2020.108361
Carroll M (2001) Innate immunity in the etiopathology of autoimmunity. Nat Immunol 2:1089–1090. https://doi.org/10.1038/ni1201-1089
Zhang H, Xu H, Ashby CR Jr, Assaraf YG, Chen ZS, Liu HM (2021) Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med Res Rev 41:525–555. https://doi.org/10.1002/med.21739
Liu YM, Chen JW, Chen LX, Xie X, Mao N (2016) Overexpression of P-glycoprotein on fibroblast-like synoviocytes in refractory rheumatoid arthritis patients: a potential mechanism for multidrug resistance in rheumatoid arthritis treatment. Genet Mol Res. https://doi.org/10.4238/gmr.15027927
Sava GP, Fan H, Fisher RA, Lusvarghi S, Pancholi S, Ambudkar SV, Martin LA, Charles Coombes R, Buluwela L, Ali S (2020) ABC-transporter upregulation mediates resistance to the CDK7 inhibitors THZ1 and ICEC0942. Oncogene 39:651–663. https://doi.org/10.1038/s41388-019-1008-y
Kang HE, Malinen MM, Saran C, Honkakoski P, Brouwer KLR (2019) Optimization of canalicular ABC transporter function in HuH-7 cells by modification of culture conditions. Drug Metab Dispos 47:1222–1230. https://doi.org/10.1124/dmd.119.087676
Zolnerciks JK, Andress EJ, Nicolaou M, Linton KJ (2011) Structure of ABC transporters. Essays Biochem 50:43–61. https://doi.org/10.1042/bse0500043
Ling V, Thompson LH (1974) Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol 83:103–116. https://doi.org/10.1002/jcp.1040830114
Arana MR, Altenberg GA (2019) ATP-binding cassette exporters: structure and mechanism with a focus on p-glycoprotein and MRP1. Curr Med Chem 26:1062–1078. https://doi.org/10.2174/0929867324666171012105143
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58. https://doi.org/10.1038/nrc706
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398. https://doi.org/10.1146/annurev.pharmtox.39.1.361
Matsson P, Pedersen JM, Norinder U, Bergstrom CA, Artursson P (2009) Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm Res 26:1816–1831. https://doi.org/10.1007/s11095-009-9896-0
Raub TJ (2006) P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol Pharm 3:3–25. https://doi.org/10.1021/mp0500871
Wang RB, Kuo CL, Lien LL, Lien EJ (2003) Structure-activity relationship: analyses of p-glycoprotein substrates and inhibitors. J Clin Pharm Ther 28:203–228. https://doi.org/10.1046/j.1365-2710.2003.00487.x
Cascorbi I (2011) P-glycoprotein: tissue distribution, substrates, and functional consequences of genetic variations. Handb Exp Pharmacol. https://doi.org/10.1007/978-3-642-14541-4_6
Soldner ELB, Hartz AMS, Akanuma SI, Pekcec A, Doods H, Kryscio RJ, Hosoya KI, Bauer B (2019) Inhibition of human microsomal PGE2 synthase-1 reduces seizure-induced increases of P-glycoprotein expression and activity at the blood-brain barrier. FASEB J 33:13966–13981. https://doi.org/10.1096/fj.201901460RR
Staud F, Ceckova M, Micuda S, Pavek P (2010) Expression and function of p-glycoprotein in normal tissues: effect on pharmacokinetics. Methods Mol Biol 596:199–222. https://doi.org/10.1007/978-1-60761-416-6_10
Li XQ, Wang L, Lei Y, Hu T, Zhang FL, Cho CH, To KK (2015) Reversal of P-gp and BCRP-mediated MDR by tariquidar derivatives. Eur J Med Chem 101:560–572. https://doi.org/10.1016/j.ejmech.2015.06.049
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650–1654. https://doi.org/10.1126/science.1360704
Barrand MA, Bagrij T, Neo SY (1997) Multidrug resistance-associated protein: a protein distinct from P-glycoprotein involved in cytotoxic drug expulsion. Gen Pharmacol 28:639–645. https://doi.org/10.1016/s0306-3623(96)00284-4
Leslie EM, Deeley RG, Cole SP (2001) Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology 167:3–23. https://doi.org/10.1016/s0300-483x(01)00454-1
Stride BD, Valdimarsson G, Gerlach JH, Wilson GM, Cole SP, Deeley RG (1996) Structure and expression of the messenger RNA encoding the murine multidrug resistance protein, an ATP-binding cassette transporter. Mol Pharmacol 49:962–971
Bakos E, Hegedus T, Hollo Z, Welker E, Tusnady GE, Zaman GJ, Flens MJ, Varadi A, Sarkadi B (1996) Membrane topology and glycosylation of the human multidrug resistance-associated protein. J Biol Chem 271:12322–12326. https://doi.org/10.1074/jbc.271.21.12322
Wu YJ, Wang C, Wei W (2018) The effects of DMARDs on the expression and function of P-gp, MRPs, BCRP in the treatment of autoimmune diseases. Biomed Pharmacother 105:870–878. https://doi.org/10.1016/j.biopha.2018.06.015
Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 92:1295–1302. https://doi.org/10.1093/jnci/92.16.1295
Borst P, Evers R, Kool M, Wijnholds J (1999) The multidrug resistance protein family. Biochim Biophys Acta 1461:347–357. https://doi.org/10.1016/s0005-2736(99)00167-4
Hooijberg JH, Broxterman HJ, Kool M, Assaraf YG, Peters GJ, Noordhuis P, Scheper RJ, Borst P, Pinedo HM, Jansen G (1999) Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res 59:2532–2535
Loe DW, Almquist KC, Deeley RG, Cole SP (1996) Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem 271:9675–9682. https://doi.org/10.1074/jbc.271.16.9675
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95:15665–15670. https://doi.org/10.1073/pnas.95.26.15665
Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP (2017) Structure of the human multidrug transporter ABCG2. Nature 546:504–509. https://doi.org/10.1038/nature22345
Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE (2006) Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett 235:84–92. https://doi.org/10.1016/j.canlet.2005.04.024
Kirtane AR, Kalscheuer SM, Panyam J (2013) Exploiting nanotechnology to overcome tumor drug resistance: challenges and opportunities. Adv Drug Deliv Rev 65:1731–1747. https://doi.org/10.1016/j.addr.2013.09.001
Polgar O, Robey RW, Bates SE (2008) ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol 4:1–15. https://doi.org/10.1517/17425255.4.1.1
Kechida M (2019) Update on autoimmune diseases pathogenesis. Curr Pharm Des 25:2947–2952. https://doi.org/10.2174/1381612825666190709205421
Yang SH, Gao CY, Li L, Chang C, Leung PSC, Gershwin ME, Lian ZX (2018) The molecular basis of immune regulation in autoimmunity. Clin Sci (Lond) 132:43–67. https://doi.org/10.1042/CS20171154
Cooper GS, Stroehla BC (2003) The epidemiology of autoimmune diseases. Autoimmun Rev 2:119–125. https://doi.org/10.1016/s1568-9972(03)00006-5
Zhang L, Yu J, Wang C, Wei W (2019) The effects of total glucosides of paeony (TGP) and paeoniflorin (Pae) on inflammatory-immune responses in rheumatoid arthritis (RA). Funct Plant Biol 46:107–117. https://doi.org/10.1071/FP18080
Long H, Yin H, Wang L, Gershwin ME, Lu Q (2016) The critical role of epigenetics in systemic lupus erythematosus and autoimmunity. J Autoimmun 74:118–138. https://doi.org/10.1016/j.jaut.2016.06.020
Shaheen IA, Botros SK, Morgan DS (2014) Detection of expression of IL-18 and its binding protein in Egyptian pediatric immune thrombocytopenic purpura. Platelets 25:193–196. https://doi.org/10.3109/09537104.2013.784734
Janikashvili N, Samson M, Magen E, Chikovani T (2016) Immunotherapeutic targeting in autoimmune diseases. Mediators Inflamm 2016:1432702. https://doi.org/10.1155/2016/1432702
Yasuda S, Kono M, Shimamura S, Kurita T, Odani T, Atsumi T (2016) Prognosis and progress in immunotherapies for organ involvements in systemic autoimmune diseases. Nihon Rinsho Meneki Gakkai Kaishi 39:8–17. https://doi.org/10.2177/jsci.39.8
Fukuda Y, Lian S, Schuetz JD (2015) Leukemia and ABC transporters. Adv Cancer Res 125:171–196. https://doi.org/10.1016/bs.acr.2014.10.006
Li Y, Sun J, Gao S, Hu H, Xie P (2018) HOXB4 knockdown enhances the cytotoxic effect of paclitaxel and cisplatin by downregulating ABC transporters in ovarian cancer cells. Gene 663:9–16. https://doi.org/10.1016/j.gene.2018.04.033
Ostrowska M, Maslinski W, Prochorec-Sobieszek M, Nieciecki M, Sudol-Szopinska I (2018) Cartilage and bone damage in rheumatoid arthritis. Reumatologia 56:111–120. https://doi.org/10.5114/reum.2018.75523
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Muller-Ladner U, Mysler EF, da Silva JAP, Poor G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2019-216655
Inoue K, Yuasa H (2014) Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab Pharmacokinet 29:12–19. https://doi.org/10.2133/dmpk.dmpk-13-rv-119
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM (2018) Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18:452–464. https://doi.org/10.1038/s41568-018-0005-8
Maillefert JF, Maynadie M, Tebib JG, Aho S, Walker P, Chatard C, Dulieu V, Bouvier M, Carli PM, Tavernier C (1996) Expression of the multidrug resistance glycoprotein 170 in the peripheral blood lymphocytes of rheumatoid arthritis patients. The percentage of lymphocytes expressing glycoprotein 170 is increased in patients treated with prednisolone. Br J Rheumatol 35:430–435. https://doi.org/10.1093/rheumatology/35.5.430
Pascual-Ramos V, Atisha-Fregoso Y, Lima G, Fragoso-Loyo H, Jakez-Ocampo J, Contreras-Yanez I, Llorente L (2016) Rheumatoid arthritis (RA) disease activity predicts function of ABCB1 (P-gp) and ABCG2 (BCRP1) drug-efflux transporters. Gac Med Mex 152:582–586
Kuwano M, Oda Y, Izumi H, Yang SJ, Uchiumi T, Iwamoto Y, Toi M, Fujii T, Yamana H, Kinoshita H, Kamura T, Tsuneyoshi M, Yasumoto K, Kohno K (2004) The role of nuclear Y-box binding protein 1 as a global marker in drug resistance. Mol Cancer Ther 3:1485–1492
Tsujimura S, Tanaka Y (2015) Disease control by regulation of P-glycoprotein on lymphocytes in patients with rheumatoid arthritis. World J Exp Med 5:225–231. https://doi.org/10.5493/wjem.v5.i4.225
Wang J, Mao N, Xie X, Li S, Chen WJ (2019) High expression of multidrug resistance gene-1 can aggravate resistance to methotrexate in rheumatoid arthritis patients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 41:595–600. https://doi.org/10.3881/j.issn.1000-503X.10927
Perez-Guerrero EE, Gonzalez-Lopez L, Munoz-Valle JF, Vasquez-Jimenez JC, Ramirez-Villafana M, Sanchez-Rodriguez EN, Gutierrez-Urena SR, Cerpa-Cruz S, Aguilar-Chavez EA, Cardona-Munoz EG, Vazquez-Villegas ML, Saldana-Cruz AM, Rodriguez-Jimenez NA, Fajardo-Robledo NS, Gamez-Nava JI (2018) Serum P-glycoprotein level: a potential biomarker of DMARD failure in patients with rheumatoid arthritis. Inflammopharmacology. https://doi.org/10.1007/s10787-018-0529-2
Achira M, Totsuka R, Fujimura H, Kume T (2002) Tissue-specific regulation of expression and activity of P-glycoprotein in adjuvant arthritis rats. Eur J Pharm Sci 16:29–36. https://doi.org/10.1016/s0928-0987(02)00052-0
van der Heijden JW, Oerlemans R, Tak PP, Assaraf YG, Kraan MC, Scheffer GL, van der Laken CJ, Lems WF, Scheper RJ, Dijkmans BA, Jansen G (2009) Involvement of breast cancer resistance protein expression on rheumatoid arthritis synovial tissue macrophages in resistance to methotrexate and leflunomide. Arthritis Rheum 60:669–677. https://doi.org/10.1002/art.24354
Agarwal V, Mittal SK, Misra R (2009) Expression of multidrug resistance-1 protein correlates with disease activity rather than the refractoriness to methotrexate therapy in rheumatoid arthritis. Clin Rheumatol 28:427–433. https://doi.org/10.1007/s10067-008-1071-1
van der Heijden J, de Jong MC, Dijkmans BA, Lems WF, Oerlemans R, Kathmann I, Scheffer GL, Scheper RJ, Assaraf YG, Jansen G (2004) Acquired resistance of human T cells to sulfasalazine: stability of the resistant phenotype and sensitivity to non-related DMARDs. Ann Rheum Dis 63:131–137. https://doi.org/10.1136/ard.2003.006494
Tang H, Wu YJ, Xiao F, Wang B, Asenso J, Wang Y, Sun W, Wang C, Wei W (2019) Regulation of CP-25 on P-glycoprotein in synoviocytes of rats with adjuvant arthritis. Biomed Pharmacother 119:109432. https://doi.org/10.1016/j.biopha.2019.109432
Hay EM, Snaith ML (1995) ABC of rheumatology. Systemic lupus erythematosus and lupus-like syndromes. BMJ 310:1257–1261. https://doi.org/10.1136/bmj.310.6989.1257
Kansal A, Tripathi D, Rai MK, Agarwal V (2016) Persistent expression and function of P-glycoprotein on peripheral blood lymphocytes identifies corticosteroid resistance in patients with systemic lupus erythematosus. Clin Rheumatol 35:341–349. https://doi.org/10.1007/s10067-015-3079-7
Ragab SM, Soliman MA (2013) P-glycoprotein-1 functional activity in CD5+CD7+ and CD20+ lymphocytes in systemic lupus erythematosus children: relation to disease activity, complications and steroid response. Egypt J Immunol 20:101–115
Wang J, Liu Y, Zhao J, Xu J, Li S, Qin X (2017) P-glycoprotein gene MDR1 polymorphisms and susceptibility to systemic lupus erythematosus in Guangxi population: a case-control study. Rheumatol Int 37:537–545. https://doi.org/10.1007/s00296-017-3652-2
Tsujimura S, Saito K, Nakayamada S, Tanaka Y (2007) Relevance of multidrug resistance 1 and P-glycoprotein to drug resistance in patients with systemic lupus erythematosus. Histol Histopathol 22:465–468. https://doi.org/10.14670/HH-22.465
Ruiz-Irastorza G, Danza A, Khamashta M (2012) Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 51:1145–1153. https://doi.org/10.1093/rheumatology/ker410
Zhang B, Shi Y, Lei TC (2012) Detection of active P-glycoprotein in systemic lupus erythematosus patients with poor disease control. Exp Ther Med 4:705–710. https://doi.org/10.3892/etm.2012.667
Fukuda T, Brunner HI, Sagcal-Gironella AC, Vinks AA (2011) Nonsteroidal anti-inflammatory drugs may reduce enterohepatic recirculation of mycophenolic acid in patients with childhood-onset systemic lupus erythematosus. Ther Drug Monit 33:658–662. https://doi.org/10.1097/FTD.0b013e318228195f
Tsujimura S, Saito K, Tokunaga M, Nakatsuka K, Nakayamada S, Nakano K, Tanaka Y (2005) Overcoming treatment unresponsiveness mediated by P-glycoprotein overexpression on lymphocytes in refractory active systemic lupus erythematosus. Mod Rheumatol 15:28–32. https://doi.org/10.1007/s10165-004-0354-x
Chen N, Cui D, Wang Q, Wen Z, Finkelman RD, Welty D (2018) In vitro drug-drug interactions of budesonide: inhibition and induction of transporters and cytochrome P450 enzymes. Xenobiotica 48:637–646. https://doi.org/10.1080/00498254.2017.1344911
Larabi A, Barnich N, Nguyen HTT (2020) New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 16:38–51. https://doi.org/10.1080/15548627.2019.1635384
Verma N, Ahuja V, Paul J (2013) Profiling of ABC transporters during active ulcerative colitis and in vitro effect of inflammatory modulators. Dig Dis Sci 58:2282–2292. https://doi.org/10.1007/s10620-013-2636-7
Zhang YJ, Xu JJ, Wang P, Wang JL (2014) Multidrug resistance gene and its relationship to ulcerative colitis and immune status of ulcerative colitis. Genet Mol Res 13:10837–10851. https://doi.org/10.4238/2014.December.19.5
Zamek-Gliszczynski MJ, Bedwell DW, Bao JQ, Higgins JW (2012) Characterization of SAGE Mdr1a (P-gp), Bcrp, and Mrp2 knockout rats using loperamide, paclitaxel, sulfasalazine, and carboxydichlorofluorescein pharmacokinetics. Drug Metab Dispos 40:1825–1833. https://doi.org/10.1124/dmd.112.046508
Damiao A, de Azevedo MFC, Carlos AS, Wada MY, Silva TVM, Feitosa FC (2019) Conventional therapy for moderate to severe inflammatory bowel disease: a systematic literature review. World J Gastroenterol 25:1142–1157. https://doi.org/10.3748/wjg.v25.i9.1142
Onodera M, Endo K, Kakuta Y, Kuroha M, Kimura T, Hiramoto K, Kanazawa Y, Negoro K, Shiga H, Kinouchi Y, Shimosegawa T (2017) ATP-binding cassette subfamily B member 1 1236C/T polymorphism significantly affects the therapeutic outcome of tacrolimus in patients with refractory ulcerative colitis. J Gastroenterol Hepatol 32:1562–1569. https://doi.org/10.1111/jgh.13753
Onnie CM, Fisher SA, Pattni R, Sanderson J, Forbes A, Lewis CM, Mathew CG (2006) Associations of allelic variants of the multidrug resistance gene (ABCB1 or MDR1) and inflammatory bowel disease and their effects on disease behavior: a case-control and meta-analysis study. Inflamm Bowel Dis 12:263–271. https://doi.org/10.1097/01.MIB.0000209791.98866.ba
Ejaz A, Radia D (2019) Diagnosis and management of primary immune thrombocytopenia in adults. Br J Hosp Med (Lond) 80:C54–C57. https://doi.org/10.12968/hmed.2019.80.4.C54
Cines DB, Blanchette VS (2002) Immune thrombocytopenic purpura. N Engl J Med 346:995–1008. https://doi.org/10.1056/NEJMra010501
McMillan R (2007) The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol 44:S3–S11. https://doi.org/10.1053/j.seminhematol.2007.11.002
Psaila B, Bussel JB (2007) Immune thrombocytopenic purpura. Hematol Oncol Clin North Am 21(743–59):vii. https://doi.org/10.1016/j.hoc.2007.06.007
Levy AS, Cunningham-Rundles S, Mazza B, Simm M, Gorlick R, Bussel J (2002) High P-glycoprotein-mediated export observed in patients with a history of idiopathic thrombocytopenic purpura. Br J Haematol 118:836–838. https://doi.org/10.1046/j.1365-2141.2002.03709.x
Wang XY, Cai YX, Tian WH, Zhou YY (2017) Correlation studies of P-glycoprotein and lymphocyte subgroup in patients with immune thrombocytopenia. Int J Lab Med 38:1910–1915
Liu W, Li H, Zhang D, Lv M, Li Y, Hao Y, Chen Y, Liu X, Xue F, Zhang L, Yang R (2016) Effects of the multidrug resistance-1 gene on drug resistance in primary immune thrombocytopenia. Autoimmunity 49:486–495. https://doi.org/10.1080/08916934.2016.1191476
Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212:8–27. https://doi.org/10.1111/j.0105-2896.2006.00427.x
Mazzucco KL, Junior LM, Lemos NE, Wieck A, Pezzi A, Laureano AM, Amorin B, Valim V, Silla L, Daudt LE, Marostica PJ (2013) Assessment of regulatory T cells in childhood immune thrombocytopenic purpura. ISRN Hematol 2013:143687. https://doi.org/10.1155/2013/143687
Akyol Erikci A, Karagoz B, Bilgi O (2016) Regulatory T cells in patients with idiopathic thrombocytopenic purpura. Turk J Haematol 33:153–155. https://doi.org/10.4274/tjh.2015.0335
Borisov MA, Petrakova OS, Gvazava IG, Kalistratova EN, Vasiliev AV (2016) Stem cells in the treatment of insulin-dependent diabetes mellitus. Acta Naturae 8:31–43
Aleksunes LM, Xu J, Lin E, Wen X, Goedken MJ, Slitt AL (2013) Pregnancy represses induction of efflux transporters in livers of type I diabetic mice. Pharm Res 30:2209–2220. https://doi.org/10.1007/s11095-013-0981-z
Anger GJ, Cressman AM, Piquette-Miller M (2012) Expression of ABC Efflux transporters in placenta from women with insulin-managed diabetes. PLoS ONE 7:e35027. https://doi.org/10.1371/journal.pone.0035027
Zhang LL, Lu L, Jin S, Jing XY, Yao D, Hu N, Liu L, Duan R, Liu XD, Wang GJ, Xie L (2011) Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 32:956–966. https://doi.org/10.1038/aps.2011.33
Reichel V, Burghard S, John I, Huber O (2011) P-glycoprotein and breast cancer resistance protein expression and function at the blood-brain barrier and blood-cerebrospinal fluid barrier (choroid plexus) in streptozotocin-induced diabetes in rats. Brain Res 1370:238–245. https://doi.org/10.1016/j.brainres.2010.11.012
Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV (2015) DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T-cell tolerance and inhibit diabetes. Diabetes 64:3521–3531. https://doi.org/10.2337/db14-1880
Hotta-Iwamura C, Tarbell KV (2016) Type 1 diabetes genetic susceptibility and dendritic cell function: potential targets for treatment. J Leukoc Biol 100:65–80. https://doi.org/10.1189/jlb.3MR1115-500R
Yu HC, Lu MC, Li C, Huang HL, Huang KY, Liu SQ, Lai NS, Huang HB (2013) Targeted delivery of an antigenic peptide to the endoplasmic reticulum: application for development of a peptide therapy for ankylosing spondylitis. PLoS ONE 8:e77451. https://doi.org/10.1371/journal.pone.0077451
Yan RJ, Lou TT, Wu YF, Chen WS (2017) Single nucleotide polymorphisms of ABCB1 gene and response to etanercept treatment in patients with ankylosing spondylitis in a Chinese Han population. Medicine (Baltimore) 96:e5929. https://doi.org/10.1097/MD.0000000000005929
Yeremenko N, Paramarta JE, Baeten D (2014) The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol 26:361–370. https://doi.org/10.1097/BOR.0000000000000069
Bianchi E, Rogge L (2019) The IL-23/IL-17 pathway in human chronic inflammatory diseases-new insight from genetics and targeted therapies. Genes Immun 20:415–425. https://doi.org/10.1038/s41435-019-0067-y
Smith JA (2015) Update on ankylosing spondylitis: current concepts in pathogenesis. Curr Allergy Asthma Rep 15:489. https://doi.org/10.1007/s11882-014-0489-6
Wellcome Trust Case Control Centre, Australo-Anglo-American Spondylitis Centre, Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davison D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop MG, Connell J, Dominiczak A, Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Biologics in RAG, Genomics Study Syndicate Steering Centre, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hilder SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Breast Cancer Susceptibility Centre, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Newport M, Sirugo G, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Davison D, Ferreira T, Pereira-Gale J, Hallgrimsdottir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Brown MA, Compston A, Farrall M, Hall AS, Hattersley AT, Hill AV, Parkes M, Pembrey M, Stratton MR, Mitchell SL, Newby PR, Brand OJ, Carr-Smith J, Pearce SH, McGinnis R, Keniry A, Deloukas P, Reveille JD, Zhou X, Sims AM, Dowling A, Taylor J, Doan T, Davis JC, Savage L, Ward MM, Learch TL, Weisman MH, Brown M (2007) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39:1329–1337. https://doi.org/10.1038/ng.2007.17
Bianchi E, Rogge L (2019) The IL-23/IL-17 pathway in human chronic inflammatory diseases—new insight from genetics and targeted therapies. Microbes Infect 21:246–253. https://doi.org/10.1016/j.micinf.2019.06.009
Xu W, Chen S, Wang X, Tanaka S, Onda K, Sugiyama K, Yamada H, Hirano T (2021) Molecular mechanisms and therapeutic implications of tetrandrine and cepharanthine in T cell acute lymphoblastic leukemia and autoimmune diseases. Pharmacol Ther 217:107659. https://doi.org/10.1016/j.pharmthera.2020.107659
Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS (2014) Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 211:89–104. https://doi.org/10.1084/jem.20130301
Ho GT, Soranzo N, Nimmo ER, Tenesa A, Goldstein DB, Satsangi J (2006) ABCB1/MDR1 gene determines susceptibility and phenotype in ulcerative colitis: discrimination of critical variants using a gene-wide haplotype tagging approach. Hum Mol Genet 15:797–805. https://doi.org/10.1093/hmg/ddi494
Barnes PJ, Adcock IM (2009) Glucocorticoid resistance in inflammatory diseases. Lancet 373:1905–1917. https://doi.org/10.1016/S0140-6736(09)60326-3
Hodge G, Hodge S, Nguyen PT, Yeo A, Sarkar P, Badiei A, Holmes-Liew CL, Reynolds PN, Holmes M (2018) Bronchiolitis obliterans syndrome is associated with increased p-glycoprotein expression and loss of glucocorticoid receptor from steroid-resistant proinflammatory CD8(+) T cells. Clin Exp Immunol 192:242–250. https://doi.org/10.1111/cei.13103
Hafeez U, Gan HK, Scott AM (2018) Monoclonal antibodies as immunomodulatory therapy against cancer and autoimmune diseases. Curr Opin Pharmacol 41:114–121. https://doi.org/10.1016/j.coph.2018.05.010
Zuercher AW, Spirig R, Baz Morelli A, Rowe T, Kasermann F (2019) Next-generation Fc receptor-targeting biologics for autoimmune diseases. Autoimmun Rev 18:102366. https://doi.org/10.1016/j.autrev.2019.102366
Jain A, Irizarry-Caro RA, McDaniel MM, Chawla AS, Carroll KR, Overcast GR, Philip NH, Oberst A, Chervonsky AV, Katz JD, Pasare C (2020) T cells instruct myeloid cells to produce inflammasome-independent IL-1beta and cause autoimmunity. Nat Immunol 21:65–74. https://doi.org/10.1038/s41590-019-0559-y
Gonzalez-Figueroa P, Roco JA, Papa I, Nunez Villacis L, Stanley M, Linterman MA, Dent A, Canete PF, Vinuesa CG (2021) Follicular regulatory T cells produce neuritin to regulate B cells. Cell 184(1775–1789):e19. https://doi.org/10.1016/j.cell.2021.02.027
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 82004180), the Anhui Medical University Clinical Pharmacology and Pharmacology Co-construction Project in 2020, the Key Project Foundation of Natural Science Research in Universities of Anhui Province in China (No. KJ2021A0329), and the Foundation of Anhui Medical University (Grant No. 2018xkj065).
Author information
Authors and Affiliations
Contributions
JY and HC contributed to the literature review and data collection. JMX contributed to the grammar checking. JY and PZ contributed to the overall design and article writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they had no actual or potential competing financial interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, J., Chen, H., Xu, J. et al. Research advances in the role and pharmaceuticals of ATP-binding cassette transporters in autoimmune diseases. Mol Cell Biochem 477, 1075–1091 (2022). https://doi.org/10.1007/s11010-022-04354-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04354-y