Abstract
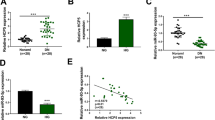
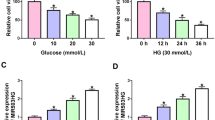
The dysregulated long noncoding RNAs (lncRNAs) are associated with the pathogenesis of diabetic nephropathy (DN). LncRNA potassium voltage-gated channel subfamily Q member 1 overlapping transcript 1 (KCNQ1OT1) plays an important role in diabetes, but the role and mechanism of KCNQ1OT1 in DN are largely unknown. Serum samples were collected from 30 DN patients and normal volunteers. High glucose (HG)-challenged human mesangial cells (HMCs) were used as a cell model of DN. KCNQ1OT1, microRNA-18b (miR-18b), and high mobility group protein A2 (HMGA2) abundances were examined via quantitative reverse transcription polymerase chain reaction or western blot. Cell proliferation was assessed via 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide. Oxidative stress was assessed via the levels of reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), and SOD2. Extracellular matrix (ECM) accumulation was investigated by the levels of fibronectin (FN), collagen I (Col I), and Col IV. The relationship between miR-18b and KCNQ1OT1 or HMGA2 was determined via dual-luciferase reporter analysis, RNA immunoprecipitation, and pull-down. KCNQ1OT1 expression was increased and miR-18b expression was decreased in DN patients and HG-challenged HMCs. miR-18b was targeted via KCNQ1OT1. Knockdown of KCNQ1OT1 weakened HG-caused proliferation, oxidative stress, and ECM accumulation of HMCs by increasing miR-18b. HMGA2 was targeted via miR-18b. miR-18b alleviated HG-induced cell proliferation, oxidative stress, and ECM accumulation by decreasing HMGA2. Silence of KCNQ1OT1 reduced HMGA2 expression via miR-18b. KCNQ1OT1 knockdown attenuated HG-induced proliferation, oxidative stress, and ECM accumulation of HMCs by regulating miR-18b/HMGA2 axis.






Similar content being viewed by others
References
Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME (2015) Diabetic kidney disease. Nat Rev Dis Primers 1:15018. https://doi.org/10.1038/nrdp.2015.18
Fineberg D, Jandeleit-Dahm KA, Cooper ME (2013) Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol 9:713–23. https://doi.org/10.1038/nrendo.2013.184
Dugbartey GJ (2017) Diabetic nephropathy: a potential savior with 'rotten-egg' smell. Pharmacol Rep 69:331–339. https://doi.org/10.1016/j.pharep.2016.11.004
Tung CW, Hsu YC, Shih YH, Chang PJ, Lin CL (2018) Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology (Carlton) 23(Suppl 4):32–37. https://doi.org/10.1111/nep.13451
Alvarez ML, Distefano JK (2013) The role of non-coding RNAs in diabetic nephropathy: potential applications as biomarkers for disease development and progression. Diabetes Res Clin Pract 99:1–11. https://doi.org/10.1016/j.diabres.2012.10.010
Leti F, Morrison E, DiStefano JK (2017) Long noncoding RNAs in the pathogenesis of diabetic kidney disease: implications for novel therapeutic strategies. Per Med 14:271–278. https://doi.org/10.2217/pme-2016-0107
Shao J, Pan X, Yin X, Fan G, Tan C, Yao Y, Xin Y, Sun C (2019) KCNQ1OT1 affects the progression of diabetic retinopathy by regulating miR-1470 and epidermal growth factor receptor. J Cell Physiol 234:17269–17279. https://doi.org/10.1002/jcp.28344
Yang F, Qin Y, Wang Y, Li A, Lv J, Sun X, Che H, Han T, Meng S, Bai Y, Wang L (2018) LncRNA KCNQ1OT1 mediates pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem 50:1230–1244. https://doi.org/10.1159/000494576
Li Y, Huo C, Lin X, Xu J (2018) Computational identification of cross-talking ceRNAs. Adv Exp Med Biol 1094:97–108. https://doi.org/10.1007/978-981-13-0719-5_10
Simpson K, Wonnacott A, Fraser DJ, Bowen T (2016) MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Curr Diab Rep 16:35. https://doi.org/10.1007/s11892-016-0724-8
Wu JH, Wang YH, Wang W, Shen W, Sang YZ, Liu L, Chen CM (2016) MiR-18b suppresses high-glucose-induced proliferation in HRECs by targeting IGF-1/IGF1R signaling pathways. Int J Biochem Cell Biol 73:41–52. https://doi.org/10.1016/j.biocel.2016.02.002
Zhang J, Cao X, Wang S, Aizimaiti M, Xielifu R, Liu J (2018) LincRNA-p21 sponges miR-18b to promote the progression of diabetic nephropathy. Am J Transl Res 10:1481–1489
Alkayyali S, Lajer M, Deshmukh H, Ahlqvist E, Colhoun H, Isomaa B, Rossing P, Groop L, Lyssenko V (2013) Common variant in the HMGA2 gene increases susceptibility to nephropathy in patients with type 2 diabetes. Diabetologia 56:323–9. https://doi.org/10.1007/s00125-012-2760-5
Li Y, Zheng L, Huang D, Cao H, Gao Y, Fan Z (2020) LNCRNA CDKN2B-AS1 regulates mesangial cell proliferation and extracellular matrix accumulation via miR-424-5p/HMGA2 axis. Biomed. Pharmacother. 121:109622. https://doi.org/10.1016/j.biopha.2019.109622
Chen B, Li Y, Liu Y, Xu Z (2019) circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J. Cell. Physiol. 234:21249–21259. https://doi.org/10.1002/jcp.28730
Zhang X, Zhu H, Zhang Y, Zhang C, Jiao J, Xing X (2020) LncRNA CASC2 regulates high glucose-induced proliferation, extracellular matrix accumulation and oxidative stress of human mesangial cells via miR-133b/FOXP1 axis. Eur Rev Med Pharmacol Sci 24:802–812. https://doi.org/10.26355/eurrev_202001_20063
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–8. https://doi.org/10.1006/meth.2001.1262
Mazumdar A, Haddad Y, Milosavljevic V, Michalkova H, Guran R, Bhowmick S, Moulick A (2020) Peptide-carbon quantum dots conjugate, derived from human retinoic acid receptor responder protein 2, against antibiotic-resistant gram positive and gram negative pathogenic bacteria. Nanomaterials (Basel). https://doi.org/10.3390/nano10020325
Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, Zhou H, Cai JH (2018) Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci 22:3826–3836. https://doi.org/10.26355/eurrev_201806_15267
Wei W, Peng J, Li J (2019) Curcumin attenuates hypoxia/reoxygenationinduced myocardial injury. Mol Med Rep 20:4821–4830. https://doi.org/10.3892/mmr.2019.10742
Persson F, Rossing P (2018) (2018) Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int Suppl 2011(8):2–7. https://doi.org/10.1016/j.kisu.2017.10.003
Ilyas Z, Chaiban J, Krikorian A (2017) Novel insights into the pathophysiology and clinical aspects of diabetic nephropathy. Rev Endocr Metab Disord 18:21–28. https://doi.org/10.1007/s11154-017-9422-3
Li Y, Xu K, Xu K, Chen S, Cao Y, Zhan H (2019) Roles of identified long noncoding rna in diabetic nephropathy. J Diabetes Res 2019:5383010. https://doi.org/10.1155/2019/5383010
Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, Jiang Y, Li A, Sun X, Yue E, Ren L, Li Y, Bai Y, Wang L (2018) Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis 9:1000. https://doi.org/10.1038/s41419-018-1029-4
Wolf G, Ziyadeh FN (1999) Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 56:393–405. https://doi.org/10.1046/j.1523-1755.1999.00590.x
Kolset SO, Reinholt FP, Jenssen T (2012) Diabetic nephropathy and extracellular matrix. J Histochem Cytochem 60:976–86. https://doi.org/10.1369/0022155412465073
Sagoo MK, Gnudi L (2018) Diabetic nephropathy: is there a role for oxidative stress? Free Radic Biol Med 116:50–63. https://doi.org/10.1016/j.freeradbiomed.2017.12.040
Li K, Zhai M, Jiang L, Song F, Zhang B, Li J, Li H, Li B, Xia L, Xu L, Cao Y, He M, Zhu H, Zhang L, Liang H, Jin Z, Duan W, Wang S (2019) Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxid Med Cell Longev 2019:6746907. https://doi.org/10.1155/2019/6746907
Li Y, Li Y, Zheng S (2020) Inhibition of NADPH oxidase 5 (NOX5) suppresses high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in human glomerular mesangial cells. Med Sci Monit 26:e919399. https://doi.org/10.12659/MSM.919399
Zhu XJ, Gong Z, Li SJ, Jia HP, Li DL (2019) Long non-coding RNA Hottip modulates high-glucose-induced inflammation and ECM accumulation through miR-455-3p/WNT2B in mouse mesangial cells. Int J Clin Exp Pathol 12:2435–2445
Chen B, Li Y, Liu Y, Xu Z (2019) circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol 234:21249–21259. https://doi.org/10.1002/jcp.28730
Ulitsky I (2018) Interactions between short and long noncoding RNAs. FEBS Lett 592:2874–2883. https://doi.org/10.1002/1873-3468.13085
Xie Y, Wang M, Shao Y, Deng X, Chen Y (2019) Long non-coding RNA KCNQ1OT1 Contributes To Antiepileptic Drug Resistance Through the miR-138-5p/ABCB1 axis in vitro. Front Neurosci 13:1358. https://doi.org/10.3389/fnins.2019.01358
Qiao CY, Qiao TY, Jin H, Liu LL, Zheng MD, Wang ZL (2020) LncRNA KCNQ1OT1 contributes to the cisplatin resistance of tongue cancer through the KCNQ1OT1/miR-124-3p/TRIM14 axis. Eur Rev Med Pharmacol Sci 24:200–212. https://doi.org/10.26355/eurrev_202001_19912
Chen L, Xiong Y, Yan C, Zhou W, Endo Y, Xue H, Hu Y, Hu L, Leng X, Liu J, Lin Z, Mi B, Liu G (2020) LncRNA KCNQ1OT1 accelerates fracture healing via modulating miR-701-3p/FGFR3 axis. FASEB J. https://doi.org/10.1096/fj.201901864RR
Wang T, Zhu H, Yang S, Fei X (2019) Let7a5p may participate in the pathogenesis of diabetic nephropathy through targeting HMGA2. Mol Med Rep 19:4229–4237. https://doi.org/10.3892/mmr.2019.10057
Azushima K, Gurley SB, Coffman TM (2018) Modelling diabetic nephropathy in mice. Nat Rev Nephrol 14:48–56. https://doi.org/10.1038/nrneph.2017.142
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Li, M. & Bai, L. KCNQ1OT1/miR-18b/HMGA2 axis regulates high glucose-induced proliferation, oxidative stress, and extracellular matrix accumulation in mesangial cells. Mol Cell Biochem 476, 321–331 (2021). https://doi.org/10.1007/s11010-020-03909-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03909-1