Abstract
Background
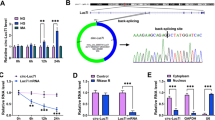
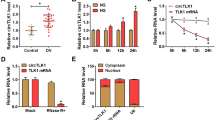
Circular RNA (circRNA) is widely shown to be associated with the development of diabetic nephropathy (DN). Our study aimed to further explore the role of circ_0000064 and provide a new mechanism for its action in DN.
Methods
Cell models of DN in vitro were constructed by treating human renal mesangial cells (HRMCs) with high glucose (HG). The expression of circ_0000064, microRNA-424-5p (miR-424-5p) and Wnt family member 2B (WNT2B) mRNA was detected by quantitative real-time PCR (qPCR). Cell proliferation was assessed by CCK-8 assay and EdU assay. Cell cycle was characterized by DNA content using flow cytometry. The releases of pro-inflammatory factors were checked using commercial ELISA kits. The expression of cell cycle- and fibrosis-associated proteins was detected by western blot. The interplays between miR-424-5p and circ_0000064 or WNT2B were verified by dual-luciferase reporter assay and RIP assay.
Results
Circ_0000064 and WNT2B were upregulated, while miR-424-5p was downregulated in HG-treated HRMCs. Circ_0000064 knockdown largely attenuated HG-induced proliferation, inflammatory responses and extracellular matrix (ECM) accumulation in HRMCs, and miR-424-5p deficiency reversed the role of circ_0000064 knockdown. MiR-424-5p was a target of circ_0000064, and miR-424-5p directly bound to WNT2B. MiR-424-5p restoration alleviated HG-induced proliferation, inflammatory responses and ECM accumulation in HRMCs, and WNT2B overexpression partially abolished the effects of miR-424-5p.
Conclusion
Circ_0000064 knockdown ameliorated HG-induced HRMC dysfunctions through miR-424-5p enrichment-mediated WNT2B inhibition, hinting that circ_0000064 contributed to DN development.








Similar content being viewed by others
Data availability
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
References
Dounousi E, et al. Improvements in the management of diabetic nephropathy. Rev Diabet Stud. 2015;12(1–2):119–33.
Cooper ME. Diabetes: treating diabetic nephropathy-still an unresolved issue. Nat Rev Endocrinol. 2012;8(9):515–6.
Badal SS, Danesh FR. New insights into molecular mechanisms of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 Suppl 2):S63-83.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14.
Loganathan TS, et al. Interactions among non-coding RNAs in diabetic nephropathy. Front Pharmacol. 2020;11:191.
Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17(4):205–11.
Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32(5):453–61.
Chen B, et al. circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol. 2019;234(11):21249–59.
Danesh FR, et al. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/ p21 signaling pathway: Implications for diabetic nephropathy. Proc Natl Acad Sci U S A. 2002;99(12):8301–5.
Sharma, K. and F.N. Ziyadeh, Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes, 1995. 44(10): p. 1139–46.
Hu W, et al. Circular RNA circRNA_15698 aggravates the extracellular matrix of diabetic nephropathy mesangial cells via miR-185/TGF-beta1. J Cell Physiol. 2019;234(2):1469–76.
Luo YH, et al. Emerging roles of circular RNA hsa_circ_0000064 in the proliferation and metastasis of lung cancer. Biomed Pharmacother. 2017;96:892–8.
Ge X, et al. Circular RNA Circ_0000064 promotes the proliferation and fibrosis of mesangial cells via miR-143 in diabetic nephropathy. Gene. 2020;758: 144952.
Hansen TB, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8.
Sun Q, et al. Differential Expression and Bioinformatics Analysis of circRNA in Non-small Cell Lung Cancer. Front Genet. 2020;11: 586814.
Wang G, et al. Upregulation of microRNA-424 relieved diabetic nephropathy by targeting Rictor through mTOR Complex2/Protein Kinase B signaling. J Cell Physiol. 2019;234(7):11646–53.
Zhu XJ, et al. Long non-coding RNA Hottip modulates high-glucose-induced inflammation and ECM accumulation through miR-455-3p/WNT2B in mouse mesangial cells. Int J Clin Exp Pathol. 2019;12(7):2435–45.
Zhang J, et al. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16(1):151.
Arora MK, Singh UK. Oxidative stress: meeting multiple targets in pathogenesis of diabetic nephropathy. Curr Drug Targets. 2014;15(5):531–8.
Wang W, et al. Circ_0123996 promotes cell proliferation and fibrosisin mouse mesangial cells through sponging miR-149-5p and inducing Bach1 expression. Gene. 2020;761: 144971.
Wu L, et al. Circ_0000064 adsorption of microRNA-143 promotes malignant progression of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2019;23(21):9321–30.
Saaoud F, et al. Circular RNAs are a novel type of non-coding RNAs in ROS regulation, cardiovascular metabolic inflammations and cancers. Pharmacol Ther. 2021;220: 107715.
Long F, et al. Comprehensive landscape and future perspectives of circular RNAs in colorectal cancer. Mol Cancer. 2021;20(1):26.
Feng ZH, et al. EIF4A3-induced circular RNA PRKAR1B promotes osteosarcoma progression by miR-361-3p-mediated induction of FZD4 expression. Cell Death Dis. 2021;12(11):1025.
Wei Y, et al. EIF4A3-induced circular RNA ASAP1 promotes tumorigenesis and temozolomide resistance of glioblastoma via NRAS/MEK1/ERK1-2 signaling. Neuro Oncol. 2021;23(4):611–24.
Zhang C, et al. Circular RNA circPPM1F modulates M1 macrophage activation and pancreatic islet inflammation in type 1 diabetes mellitus. Theranostics. 2020;10(24):10908–24.
Barutta F, et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS ONE. 2013;8(11): e73798.
Li Y, et al. LNCRNA CDKN2B-AS1 regulates mesangial cell proliferation and extracellular matrix accumulation via miR-424-5p/HMGA2 axis. Biomed Pharmacother. 2020;121: 109622.
Pulkkinen K, Murugan S, Vainio S. Wnt signaling in kidney development and disease. Organogenesis. 2008;4(2):55–9.
Chang J, et al. Long non-coding RNA CDKN2B-AS1 regulates high glucose-induced human mesangial cell injury via regulating the miR-15b-5p/WNT2B axis. Diabetol Metab Syndr. 2020;12(1):109.
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial conflicts of interest.
Ethics approval and consent to participate
The present study was approved by the ethical review committee of Liuzhou People’s Hospital. Written informed consent was obtained from all enrolled patients.
Consent for publication
Patients agreed to participate in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, J., Min, Y. & Zhao, Q. Circ_0000064 knockdown attenuates high glucose-induced proliferation, inflammation and extracellular matrix deposition of mesangial cells through miR-424-5p-mediated WNT2B inhibition in cell models of diabetic nephropathy. Clin Exp Nephrol 26, 943–954 (2022). https://doi.org/10.1007/s10157-022-02241-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02241-w