Abstract
Prostate cancer (PC) is the most common reproductive cancer in men and the third leading cause of cancer death among men worldwide. Recently targeted therapy showed a significant therapeutic effect on PC, whereas finding more PC therapeutic target is still urgently needed. Melanoma-associated antigen-encoding C2 (MAGE-C2/CT10), which have significant homology with the MAGE-C1/CT-7 gene, was known to be involved in the development of a variety of tumors. However, the role and mechanism of MAGE-C2/CT10 in prostate cancer remains unclear. Herein, we found the high levels of MAGE-C2/CT10 in highly metastatic prostate cancer. Our findings confirmed that the depletion of MAGE-C2/CT10 suppressed the growth of PC cells, and restrained PC cell migration and invasion in vitro. We noticed MAGE-C2/CT10 could stimulate c-Myc expression via FBP1, and further contributed to PC cell proliferation and motility. Performing in vivo assays, we demonstrated MAGE-C2/CT10 promoted tumor growth and metastasis of PC cells in mice. Collectively, we found the abnormal expression of MAGE-C2/CT10 in PC, and revealed the regulatory mechanism underlying MAGE-C2/CT10 promoting PC progression and metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the most common reproductive cancer in men and the third leading cause of cancer death among men worldwide [1]. Although treatment options such as radical prostatectomy and radiotherapy can successfully cure most patients, about 30–40% of patients will relapse, which is the main factor affecting the survival of patients with prostate cancer [2]. In the case of advanced prostate cancer, due to its high metastasis, chemoradiotherapy and surgical resection are not effective, further increasing the mortality rate [3]. In decades, targeted therapy, as a precision therapy, has a significant therapeutic effect on prostate cancer, which is worthy of further study [4]. However, it is urgent to understand the pathogenesis of PC and find more targets for PC treatment.
In most cases, the cancer/testis (CT) antigen is expressed only in the germ cells of the human testis, whereas the CT antigen (CT-X) corresponding to the X chromosome is expressed in different tumor tissues and is more common in high-grade and advanced tumors, including lung, melanoma, bladder, and ovarian cancer [5, 6]. In recent years, a novel antibody of the CT antigen Melanoma-associated antigen-encoding C2 (MAGE-C2)/CT10 has been found to have significant homology with the MAGE-C1/CT-7 gene, and both are close to chromosome Xq27.13, which has now been shown to be involved in the development of a variety of tumors [7]. MAGE-C2, for example, promoted Warburg effects and hepatocellular carcinoma (HCC) progression [8]; MAGE-C2/CT10 induced spontaneous CD4+ and CD8+ T cell responses in patients with multiple myeloma [9, 10]. Additionally, MAGE-C2 promoted growth and tumorigenicity of melanoma cells, further stimulated KAP1 phosphorylation and DNA damage repair [11].
In prostate cancer, Prikler et al. analyzed castration-resistant prostate cancer tissues and eight hormone-sensitive prostate cancer tissues and found MAGE-C2/CT10 to be negative [12]. Lucas et al. showed that 10% prostate cancer tissues was MAGE-C2/CT10 positive [7]. Another study found that MAGE-C2/CT10 was expressed in 3.3% of primary prostate cancer tissues, significantly higher expression of MAGE-C2/CT10 protein in metastatic prostate cancer and castrated prostate cancer (16.3% and 17%, respectively), and lower survival in MAGE-C2/CT10 positive patients [12]. These results suggest that MAGE-C2/CT10 might be a predictor of prostate cancer metastasis or postoperative recurrence. However, the role and mechanism of MAGE-C2/CT10 in prostate cancer remains unclear.
Interestingly, MAGE-C2 could form a complex with TRIM28 to promote fructose 1,6-bisphosphate (FBP1) degradation, thus stimulating Warburg effects and HCC progression [13, 14]. Additionally, FBP1 negatively regulated the expression of c-myc and suppressed cell survival [14]. In prostate cancer, whether MAGE-C2 could promote the expression of c-Myc through FBP1, thus promoting the survival of tumor cells remains unknown. Therefore, here we investigated the possible role of MAGE-C2 on PC progression and explore the potential regulatory mechanism.
Materials and methods
Cell culture and transfection
Human prostate cancer cell lines LNCap, DU145, PC-3M-1E8 and PC-3M-2B4 were purchased from American Type Culture Collection (ATCC). Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA), at 37 °C in humidified atmosphere (5% CO2).
For MAGE-C2 knockdown, MAGE-C2 or scrambled shRNA purchased from Santa Cruz Biotechnology were introduced into PC-3M-1E8 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and selected with puromycin. Lentiviruses were produced as manufacturer’s manuals (Clontech).
RNA extraction and quantitative real-time PCR
Trizol reagent (Takara, Otsu, Japan) were utilized for total RNA extraction from PC cells. And the RNA was then reverse-translated into cDNA. SYBR Green was utilized for the RT-qPCR. GAPDH was used as internal controls. The primers for RT-qPCR were listed as follows: MAGE-C2, 5′-AAAGTCAGCACAGCAGAGGAG-3′, and 5′-TCTTCAGGAGCAGCAGGTAAA-3′; GAPDH: 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′.
CCK-8 assays
Cell proliferation was quantified by CCK-8 (Sigma-Aldrich) assay according to the instructions of products. Briefly, cells were plated into 96-well dishes overnight. By measuring absorbance at 490 nm, cell growth curves were depicted within 4 days at different time intervals.
Colony formation assay
Cells (1 × 103 cells/well) were placed in six-well plates and became adherent overnight. The medium was changed every 3 days. To visualize cell colonies, cells were fixed with PFA and dyed with 0.1% crystal violet after 14 days. Then, colony number were quantified manually.
Transwell assay
For detection of cell invasion and migration, transwell assay was performed. The upper chambers (Corning, NY, USA) was filled with RPMI 1640 medium with Matrigel (BD Biosciences, Bedford, MA, USA) containing transfected cells and the lower chambers contained complete medium with 10% FBS addition. After incubation, upper chamber cells were induced to migrate into the lower chambers. Cells were then fixed with the application of methanol and dyed in 0.5% crystal violet. Then, fixed cells were quantified with a light microscope (Olympus Corporation, Tokyo, Japan).
Western blot
RIPA lysis buffer added with protease cocktail inhibitor (Roche, China) was used for protein extraction and then protein was separated through gel electrophoresis according to protein mass. After transferred onto PVDF membranes, protein on the membrane was incubated with the primary antibodies targeting MAGE-C2 antibody (Santa Cruz Biotechnology), Ki-67, p21, c-Myc, PCNA, N-cadherin, E-cadherin and β-actin (All from Abcam) overnight at 4 °C. Subsequently, the membrane were immersed in secondary antibody for 2 h at room temperature. The signals were captured with chemiluminescent detection system.
Immunohistochemistry
PC sections embedded in paraffin were stained with Ki-67. Paraffin sections were rehydrated by dipping in turpentine and gradient alcohol, then immersed in 3% H2O2 for 10 min at room temperature and treated with citric acid buffer. Staining photographs were obtained under the microscope.
Animal experiments
Animal experiment in this study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University for the use of animals and conducted in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines. MAGE-C2 or scrambled shRNA tumor cells were subcutaneously injected into athymic nude mice by tail vein injection (Slac Shanghai, China). The mice were monitored in tumor volume weekly. The xenograft experiment was terminated 6 weeks later and tumors were isolated. Lymph nodes were removed from each mouse, fixed and embedded in for subsequent H&E staining for histological examination.
Statistical analysis
Mean ± standard deviation (SD) was used for data exhibition. Displayed results were performed in triplet. The Student’s t-test were used to compare differences among groups. Spearman’s correlation analysis was used to determine correlation examination. *P < 0.05, **P < 0.01 versus control group. Value of P < 0.05 was considered statistically significant.
Results
MAGE-C2 expression is enhanced in aggressive PC cell lines
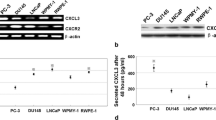
To explore the potential function of MAGE-C2 in PC, we detected the level of MAGE-C2 in human prostate cancer cell lines LNCap, DU145, PC-3M-1E8 and PC-3M-2B4. We observed higher level of MAGE-C2 in highly aggressive cell line PC-3M-1E8 compared with DU145 via qPCR and Immunoblot assays (Fig. 1a, b). And MAGE-C2 was enhanced or repressed in LNCaP or PC-3M-2B4 cells compared with DU145, according to the metastasis levels of PC cells. Thus, MAGE-C2 was induced in highly aggressive PC cell lines.
MAGE-C2 is enhanced in aggressive PC cell lines. a and b qPCR results displayed high level of MAGE-C2 in highly aggressive cell line LNCaP, PC-3M-1E8 compared with DU145 via qPCR and Immunoblot assays. And MAGE-C2 was repressed in PC-3M-2B4 cells compared with DU145 cell lines. *P < 0.05, **P < 0.01 versus control group
MAGE-C2 knockdown inhibits cell proliferation in PC cells
To explore the potential function of MAGE-C2 in PC, MAGE-C2 level was depleted by transfecting sh-MAGE-C2 plasmids into PC-3M-1E8 cells. For validation of the knockdown efficiency, we performed qPCR assay to detect MAGE-C2 level after shRNA transfection. As shown in Fig. 2a, the introduction of sh-MAGE-C2 led to a significant reduction in MAGE-C2 levels in PC-3M-1E8 cells.
MAGE-C2 inhibits proliferation, migration and invasion, and angiogenesis in PC cells. a The verification of knockdown efficiency of MAGE-C2 in PC-3M-1E8 cells. b CCK-8 assays depicted reduced cell proliferation of MAGE-C2 knockdown cells, with the decrease of relative OD values at 490 nm wavelength. c Colony formation assays revealed reduced cell colony number in MAGE-C2 knockdown cells. d PCNA and p21 protein level in control or MAGE-C2 depleted cells. *P < 0.05, **P < 0.01 versus control group
To assess the influence of MAGE-C2 on cell proliferation, we conducted CCK-8 assays and colony formation assays. We revealed that MAGE-C2 depletion in PC-3M-1E8 cells exhibited inhibitory effect on cell proliferation (Fig. 2b). Similarly, we found reduced colony number in MAGE-C2 depletion group through colony formation assay (Fig. 2c). We also found a decrease PCNA level and an increase in p21 via Immunoblot (Fig. 2d). These data suggested that MAGE-C2 regulated cell proliferation in PC cells.
MAGE-C2 knockdown inhibits cell invasion and metastasis in PC cells
For cell invasion and metastasis upon MAGE-C2 ablation, we observed MAGE-C2 knockdown inhibited wound closure in PC-3M-1E8 cells assessed by wound healing assay (Fig. 3a). And MAGE-C2 knockdown inhibited the cell migration of PC-3M-1E8 cells (Fig. 3b). Then we assessed MAGE-C2 effect on metastasis associated proteins E-cadherin and N-cadherin. Consistently, MAGE-C2 ablation induced E-cadherin expression and inhibited N-cadherin level in PC-3M-1E8 cells (Fig. 3c). These results implied MAGE-C2 acted as a key regulator in proliferation, migration and invasion in PC cells.
MAGE-C2 inhibits migration and invasion in PC cells. a Wound healing assays was performed to reveal reduced cell migration in sh MAGE-C2 group. b Transwell assay presented that MAGE-C2 promotes migration in PC cells. c Immunoblot assays displayed the E-cadherin and N-cadherin levels following MAGE-C2 depletion. *P < 0.05, **P < 0.01 versus control group
MAGE-C2 knockdown inhibits c-Myc expression in PC cells
The c-Met pathway is dysregulated in most human malignancies and regulates tumor formation and progression in PC. Thus we detect the association between MAGE-C2 and c-Myc level. We noticed MAGE-C2 knockdown downregulated c-Myc level in PC-3M-1E8 cells (Fig. 4). Thus we assumed c-Myc might be involved in MAGE-C2-regulated PC proliferation, invasion and migration.
MAGE-C2 promotes c-Myc expression by targeting FBP1
MAGE-C2 can enhance TRIM28-dependent degradation of FBP1. FBP1 loss contributes to BET inhibitors resistance by undermining c-Myc expression in pancreatic ductal adenocarcinoma. We assumed MAGE-C2 knockdown inhibited c-Myc level through regulating FBP1. To prove this, we first detected the effects of FBP1 on PC cell proliferation, migration, and invasion via CCK-8 and transwell assays. Consistent with the previous study, we found the depletion of FBP1 promoted the proliferation, migration, and invasion of PC cells in vitro (Fig. S1). We then further constructed FBP1 knockdown in MAGE-C2-depleted PC cells. Similar with our hypothesis, we discovered a reduction of c-Myc in MAGE-C2-depleted cells, while FBP1 depletion rescued c-Myc level in PC cells (Fig. 5). Taken together, our results suggested that MAGE-C2 promotes c-Myc expression by targeting FBP1.
MAGE-C2 promotes PC proliferation and metastasis via regulating c-Myc
We then performed MAGE-C2 overexpression assays and found that MAGE-C2 overexpression obviously promoted the proliferation, migration, and invasion of PC cells in vitro, via CCK-8 and transwell assays (Fig. S2). Subsequently, we explored whether MAGE-C2 regulates PC proliferation and metastasis via regulating c-Myc. We assessed cell proliferation in MAGE-C2 depletion as well as MAGE-C2 and FBP1 double depletion PC cells and revealed that FBP1 depletion could blocked the effect of MAGE-C2 depletion in cell proliferation via CCK-8 assays and colony formation assay (Fig. 6a, b). Consistently, FBP1 ablation reversed the function of sh-MAGE-C2 in the migration and invasion of PC cells via wound healing assay and transwell assay (Fig. 6c, d). Accordantly, FBP1 ablation reversed effects of MAGE-C2 knockdown on cell proliferation and migration markers (Fig. 6e). Taken together, these results led to the conclusion that MAGE-C2 induces PC cells proliferation, invasion and migration by regulating c-Myc.
MAGE-C2 promotes PC proliferation and metastasis via regulating c-Myc. a CCK-8 assay indicated MAGE-C2 knockdown effect on cell proliferation could be abrogated by FBP1 knockdown, with the decrease of relative OD values at 490 nm wavelength. b Colony formation assay revealed the reduced colony number mediated by MAGE-C2 knockdown could be abrogated by FBP1 knockdown. c Wound healing assay depicted the decreased wound closure mediated by MAGE-C2 knockdown could be abrogated by FBP1 knockdown. d Transwell assay depicted the reduced invasion ability mediated by MAGE-C2 knockdown could be reversed by FBP1 knockdown. e Change of proliferation and migration associated protein level could be altered by FBP1 knockdown assessed by Immunoblot. *P < 0.05, **P < 0.01 versus control group
MAGE-C2 knockdown inhibits tumor growth in vivo
To further detect the role of MAGE-C2 on tumorigenesis, we constructed a xenograft mouse model to evaluate tumor growth in MAGE-C2 depletion as well as control groups. We noticed that tumor volume were dramatically decreased in the sh-MAGE-C2 group relative to the sh-NC group (Fig. 7a). In addition, MAGE-C2 silencing prominently reduced the expression levels of Ki-67 (Fig. 7b). Importantly, MAGE-C2 ablation dramatically reduced lymph node metastasis (Fig. 7c). Moreover, the proliferation and invasion markers c-Myc and N-cadherin were reduced in MAGE-C2 ablated group. E-cadherin was increased in MAGE-C2 ablated group (Fig. 7d). These data indicated that knockdown of MAGE-C2 impeded tumor growth in vivo.
MAGE-C2 knockdown inhibits tumor growth in vivo.a Xenograft model manifested relatively lower tumor growth in MAGE-C2-depleted PC cells. b IHC assay depicted the reduced level of Ki-67 in control and MAGE-C2-depleted tumor. c HE assay displayed reduced lymph metastasis in MAGE-C2-depleted nude mice. d Immunoblot assay displayed MAGE-C2, c-Myc, E-cadherin, N-cadherin level in MAGE-C2-depleted nude mice. **P < 0.01 versus control group
Discussion
Most prostate cancer patients do not have obvious early symptoms, often in the physical examination accidentally found [15]. Worse, when prostate cancer progresses, surgical resection is no longer beneficial, due to its high recurrence; however, chemoradiotherapy has no significant effect on improving the prognosis of patients, and chemoradiotherapy has strong side effects [16]. In conclusion, targeted therapy is the most effective treatment for prostate cancer. A number of therapeutic targets for prostate cancer have been identified, and relevant targeted drugs have been used in clinical or in clinical trials, such as the bipolar androgen therapy [17]. In order to improve the prognosis of PC patients, more therapeutic targets still need to be found. In this study, we found that MAGE-C2/CT10 was abnormal high expression in human PC cells. Our data further confirmed the involvement of MAGE-C2/CT10 in the progression and metastasis of prostate cancer. We therefore thought MAGE-C2/CT10 could act as a possible and promising PC molecular target.
MAGE-C2 was previously identified as an antigen as well as a potential oncogenic gene that plays a role in the development of multiple tumors [11, 18]. MAGE-C2 was abnormally expressed in non-small cell lung cancer and correlated with the prognosis [18]. MAGE-C2 could also serve as a biomarker for triple-negative breast cancer, TNBC [19]. Interestingly, MAGE-C2-specific TCRs could also affect cancer progression [20]. Several microRNAs, such as miR-874, could act as a potential tumor suppressor targeting MAGE-C2 [21]. Similarly, we further provided the evidence that MAGE-C2 promoted the progression of prostate cancer. Performing CCK-8 and colony formation assays, we revealed the effects of MAGE-C2/CT10 on PC cell proliferation. Meanwhile, the promotion effects of MAGE-C2/CT10 on PC cell migration and invasion were investigated through wound healing and transwell assays in vitro. Through Immunoblot, we also noticed the effects of MAGE-C2/CT10 on the EMT of PC cells, further confirming the promotion on PC metastasis. Therefore, it was urgently needed to further study on its regulatory mechanism in different tumors.
MAGE-C2/CT10 regulated the progression of cancers in different manners [7, 11, 22]. For examples, MAGE-C2 could interact with BS69 to induce its degradation through an ubiquitin-proteasome pathway [7]. MAGE-C2 was also able to bind RBX1 and suppress ubiquitin ligase-mediated cyclin E turnover, so as to affect cancer cell cycle and progression [22]. Importantly, MAGE-C2 could interact with TRIM28 to stimulate FBP1 degradation therefore promoting the Warburg effect and HCC progression [8]. Similarly, our data further confirmed that in prostate cancer, MAGE-C2 promoted the expression of c-Myc through FBP1, and therefore contributed to PC progression and metastasis. We believed that although prostate cancer is quite different from HCC, MAGE-C2 is regulated in a similar manner, suggesting that targeted therapies developed to target MAGE-C2 may have therapeutic effects on related tumors.
In fact, FBP1 is found to play a key role in the development and progression of cancer in lung, breast, kidney and gastric cancers [23,24,25]. FBP1 promotes apoptosis of breast cancer cells by inhibiting mitochondrial autophagy [24]. It is also worth noting that the regulation of c-Myc by FBP1 has been proved. Proto-oncoprotein c-Myc is expressed in various tissues and cells, regulating the development and metastasis of prostate cancer, and is closely related to the prognosis and clinicopathological characteristics of patients [26,27,28]. Inhibiting the expression of c-Myc in prostate cancer has important clinical significance [29, 30]. Therefore, our findings exhibited that in PC cells, MAGE-C2/CT10 stimulated cancer growth and metastasis via regulating c-Myc expression. It is more clear that MAGE-C2/CT10 as a therapeutic target for PC is reasonable and promising.
In summary, we found the high expression of MAGE-C2/CT10 in human PC tissues. MAGE-C2/CT10 promoted PC cell proliferation and motility in vitro, through stimulate c-Myc expression via FBP1. Meanwhile, MAGE-C2/CT10 contributed to tumor growth and metastasis of PC cells in mice. We therefore provided the evidence that MAGE-C2/CT10 could act as a novel and promising PC molecular target.
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
10 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s11010-020-03958-6
27 July 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11010-023-04820-1
References
Zaorsky NG, Kishan AU (2020) Salvage therapy at biochemical recurrence of prostate cancer. Nat Rev Urol. https://doi.org/10.1038/s41585-020-0290-3
Ozkan A, Ucar B, Seymen H, Yildiz Yarar Y, Falay FO, Demirkol MO (2020) Posttherapeutic critical organ dosimetry of extensive 177Lu-PSMA inhibitor therapy with metastatic castration-resistant prostate cancer: one center results. Clin Nucl Med. https://doi.org/10.1097/RLU.0000000000002942
Connor MJ, Gorin MA, Ahmed HU, Nigam R (2020) Focal therapy for localized prostate cancer in the era of routine multi-parametric MRI. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/s41391-020-0206-6
Liu JM, Liu YP, Chuang HC, Wu CT, Su YL, Hsu RJ (2020) Androgen deprivation therapy for prostate cancer and the risk of hematologic disorders. PLoS ONE 15(2):e0229263. https://doi.org/10.1371/journal.pone.0229263
Grigoriadis A, Caballero OL, Hoek KS, da Silva L, Chen YT, Shin SJ, Jungbluth AA, Miller LD, Clouston D, Cebon J, Old LJ, Lakhani SR, Simpson AJ, Neville AM (2009) CT-X antigen expression in human breast cancer. Proc Natl Acad Sci USA 106(32):13493–13498. https://doi.org/10.1073/pnas.0906840106
Gao Y, Kardos J, Yang Y, Tamir TY, Mutter-Rottmayer E, Weissman B, Major MB, Kim WY, Vaziri C (2018) The cancer/testes (CT) antigen HORMAD1 promotes homologous recombinational DNA repair and radioresistance in lung adenocarcinoma cells. Sci Rep 8(1):15304. https://doi.org/10.1038/s41598-018-33601-w
Lucas S, De Plaen E, Boon T (2000) MAGE-B5, MAGE-B6, MAGE-C2, and MAGE-C3: four new members of the MAGE family with tumor-specific expression. Int J Cancer 87(1):55–60
Riener MO, Wild PJ, Soll C, Knuth A, Jin B, Jungbluth A, Hellerbrand C, Clavien PA, Moch H, Jochum W (2009) Frequent expression of the novel cancer testis antigen MAGE-C2/CT-10 in hepatocellular carcinoma. Int J Cancer 124(2):352–357. https://doi.org/10.1002/ijc.23966
de Carvalho F, Alves VL, Braga WM, Xavier CV Jr, Colleoni GW (2013) MAGE-C1/CT7 and MAGE-C2/CT10 are frequently expressed in multiple myeloma and can be explored in combined immunotherapy for this malignancy. Cancer Immunol Immunother 62(1):191–195. https://doi.org/10.1007/s00262-012-1376-4
Reinhard H, Yousef S, Luetkens T, Fehse B, Berdien B, Kroger N, Atanackovic D (2014) Cancer–testis antigen MAGE-C2/CT10 induces spontaneous CD4+ and CD8+ T-cell responses in multiple myeloma patients. Blood Cancer J 4:e212. https://doi.org/10.1038/bcj.2014.31
Bhatia N, Xiao TZ, Rosenthal KA, Siddiqui IA, Thiyagarajan S, Smart B, Meng Q, Zuleger CL, Mukhtar H, Kenney SC, Albertini MR, Jack Longley B (2013) MAGE-C2 promotes growth and tumorigenicity of melanoma cells, phosphorylation of KAP1, and DNA damage repair. J Investig Dermatol 133(3):759–767. https://doi.org/10.1038/jid.2012.355
von Boehmer L, Keller L, Mortezavi A, Provenzano M, Sais G, Hermanns T, Sulser T, Jungbluth AA, Old LJ, Kristiansen G, van den Broek M, Moch H, Knuth A, Wild PJ (2011) MAGE-C2/CT10 protein expression is an independent predictor of recurrence in prostate cancer. PLoS ONE 6(7):e21366. https://doi.org/10.1371/journal.pone.0021366
Jin X, Pan Y, Wang L, Zhang L, Ravichandran R, Potts PR, Jiang J, Wu H, Huang H (2017) MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis 6(4):e312. https://doi.org/10.1038/oncsis.2017.21
Zhang Z, Liu M, Hu Q, Xu W, Liu W, Sun Q, Ye Z, Fan G, Xu X, Yu X, Ji S, Qin Y (2019) FGFBP1, a downstream target of the FBW7/c-Myc axis, promotes cell proliferation and migration in pancreatic cancer. Am J Cancer Res 9(12):2650–2664
Iacovelli R, Ciccarese C, Schinzari G, Rossi E, Maiorano BA, Astore S, D’Angelo T, Cannella A, Pirozzoli C, Teberino MA, Pierconti F, Martini M, Tortora G (2020) Biomarkers of response to advanced prostate cancer therapy. Expert Rev Mol Diagn 20(2):195–205. https://doi.org/10.1080/14737159.2020.1707669
Lam T, Birzniece V, McLean M, Gurney H, Hayden A, Cheema BS (2020) The adverse effects of androgen deprivation therapy in prostate cancer and the benefits and potential anti-oncogenic mechanisms of progressive resistance training. Sports Med Open 6(1):13. https://doi.org/10.1186/s40798-020-0242-8
Markowski MC, Shenderov E, Eisenberger MA, Kachhap S, Pardoll DM, Denmeade SR, Antonarakis ES (2020) Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant prostate cancer. Prostate 80(5):407–411. https://doi.org/10.1002/pros.23955
Chen X, Wang L, Liu J, Huang L, Yang L, Gao Q, Shi X, Li J, Li F, Zhang Z, Zhao S, Zhang B, Van der Bruggen P, Zhang Y (2017) Expression and prognostic relevance of MAGE-A3 and MAGE-C2 in non-small cell lung cancer. Oncol Lett 13(3):1609–1618. https://doi.org/10.3892/ol.2017.5665
Zhao Q, Xu WT, Shalieer T (2016) Pilot study on MAGE-C2 as a potential biomarker for triple-negative breast cancer. Dis Markers 2016:2325987. https://doi.org/10.1155/2016/2325987
Kunert A, van Brakel M, van Steenbergen-Langeveld S, da Silva M, Coulie PG, Lamers C, Sleijfer S, Debets R (2016) MAGE-C2-specific TCRs combined with epigenetic drug-enhanced antigenicity yield robust and tumor-selective T cell responses. J Immunol 197(6):2541–2552. https://doi.org/10.4049/jimmunol.1502024
Song X, Song W, Wang Y, Wang J, Li Y, Qian X, Pang X, Zhang Y, Yin Y (2016) MicroRNA-874 functions as a tumor suppressor by targeting cancer/testis antigen HCA587/MAGE-C2. J Cancer 7(6):656–663. https://doi.org/10.7150/jca.13674
Hao J, Song X, Wang J, Guo C, Li Y, Li B, Zhang Y, Yin Y (2015) Cancer–testis antigen MAGE-C2 binds Rbx1 and inhibits ubiquitin ligase-mediated turnover of cyclin E. Oncotarget 6(39):42028–42039. https://doi.org/10.18632/oncotarget.5973
Yang C, Zhu S, Yang H, Deng S, Fan P, Li M, Jin X (2019) USP44 suppresses pancreatic cancer progression and overcomes gemcitabine resistance by deubiquitinating FBP1. Am J Cancer Res 9(8):1722–1733
Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP (2013) Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 23(3):316–331. https://doi.org/10.1016/j.ccr.2013.01.022
Li Q, Wei P, Wu J, Zhang M, Li G, Li Y, Xu Y, Li X, Xie D, Cai S, Xie K, Li D (2019) The FOXC1/FBP1 signaling axis promotes colorectal cancer proliferation by enhancing the Warburg effect. Oncogene 38(4):483–496. https://doi.org/10.1038/s41388-018-0469-8
Dong H, Hu J, Wang L, Qi M, Lu N, Tan X, Yang M, Bai X, Zhan X, Han B (2019) SOX4 is activated by C-MYC in prostate cancer. Med Oncol 36(11):92. https://doi.org/10.1007/s12032-019-1317-6
Peng F, Yang C, Kong Y, Huang X, Chen Y, Zhou Y, Xie X, Liu P (2020) CDK12 promotes breast cancer progression and maintains stemness by activating c-myc/beta-catenin Signaling. Curr Cancer Drug Targets 20(2):156–165. https://doi.org/10.2174/1568009619666191118113220
Sun W, Li J, Zhou L, Han J, Liu R, Zhang H, Ning T, Gao Z, Liu B, Chen X, Ba Y (2020) The c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in colorectal cancer. Theranostics 10(5):1981–1996. https://doi.org/10.7150/thno.37621
Lin CY, Wang BJ, Chen BC, Tseng JC, Jiang SS, Tsai KK, Shen YY, Yuh CH, Sie ZL, Wang WC, Kung HJ, Chuu CP (2019) Histone demethylase KDM4C stimulates the proliferation of prostate cancer cells via activation of AKT and c-Myc. Cancers 11:11. https://doi.org/10.3390/cancers11111785
Qu X, Sun J, Zhang Y, Li J, Hu J, Li K, Gao L, Shen L (2018) c-Myc-driven glycolysis via TXNIP suppression is dependent on glutaminase-MondoA axis in prostate cancer. Biochem Biophys Res Commun 504(2):415–421. https://doi.org/10.1016/j.bbrc.2018.08.069
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
BY conceived and designed the experiments. JQ analyzed and interpreted the results of the experiments, BY and JQ performed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
Ethics approval
Animal experiment in this study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University for the Use of Animals and conducted in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11010-023-04820-1"
The original version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, J., Yang, B. RETRACTED ARTICLE: MAGE-C2/CT10 promotes growth and metastasis through upregulating c-Myc expression in prostate cancer. Mol Cell Biochem 476, 1–10 (2021). https://doi.org/10.1007/s11010-020-03814-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03814-7