Abstract
Background
OP16, a derivative of the novel ent-kaurene diterpenoid compound separated from Rabdosia rubescens, has been confirmed for its efficacy and safety in the treatment of esophageal squamous cell carcinoma (ESCC) in our previous study. However, the precise mechanisms of tumor lethality mediated by OP16 have not yet been fully characterized.
Aims
To investigate the effects and molecular mechanism of OP16 on autophagy and apoptosis of ESCC cells.
Methods
Effects and mechanism of OP16 on autophagy of ESCC cells were first detected by Western blot, immunofluorescence, mRFP-GFP-LC3 adenovirus infection and transmission electron microscope. Next, function of autophagy and apoptosis induced by OP16 on cell death was investigated by flow cytometry and CCK-8 assay. Finally, molecular mechanism of OP16 affecting autophagy and apoptosis of ESCC cells was explored by Western blot.
Results
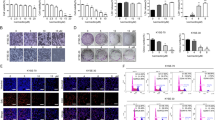
We demonstrated that OP16 could induce autophagy by promoting the formation of autophagosome and autolysosome, and promote autophagic cell death in ESCC. Furthermore, we also found that OP16 could promote cell apoptosis by activating mitochondria apoptosis pathway in ESCC. Finally, we demonstrated that OP16 affecting autophagy and mitochondria apoptosis pathway was mediated by phosphorylation of Akt.
Conclusion
Our data show that OP16 could promote cell death through affecting autophagy and mitochondria apoptosis pathway mediated by Akt in ESCC, which enriches the theoretical mechanism of anti-tumor effects of OP16 and provides a basis for treatment of OP16 on ESCC.





Similar content being viewed by others
References
Choi AM, Ryter SW, Levine B (2013) Autophagy in human health and disease. N Engl J Med 368(7):651–662. https://doi.org/10.1056/NEJMra1205406
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. https://doi.org/10.1146/annurev-genet-102808-114910
Nguyen TN, Padman BS, Usher J, Oorschot V, Ramm G, Lazarou M (2016) Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol 215(6):857–874. https://doi.org/10.1083/jcb.201607039
Huang Y, Hou JK, Chen TT, Zhao XY, Yan ZW, Zhang J, Yang J, Kogan SC, Chen GQ (2011) PML-RARalpha enhances constitutive autophagic activity through inhibiting the Akt/mTOR pathway. Autophagy 7(10):1132–1144. https://doi.org/10.4161/auto.7.10.16636
Reggiori F, Ungermann C (2017) Autophagosome Maturation and Fusion. J Mol Biol 429(4):486–496. https://doi.org/10.1016/j.jmb.2017.01.002
Ge L, Baskaran S, Schekman R, Hurley JH (2014) The protein-vesicle network of autophagy. Curr Opin Cell Biol 29:18–24. https://doi.org/10.1016/j.ceb.2014.02.005
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171(4):603–614. https://doi.org/10.1083/jcb.200507002
Amaravadi R, Kimmelman AC, White E (2016) Recent insights into the function of autophagy in cancer. Genes Dev 30(17):1913–1930. https://doi.org/10.1101/gad.287524.116
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075. https://doi.org/10.1038/nature06639
White E (2015) The role for autophagy in cancer. J Clin Invest 125(1):42–46. https://doi.org/10.1172/JCI73941
White E, DiPaola RS (2009) The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 15(17):5308–5316. https://doi.org/10.1158/1078-0432.CCR-07-5023
Hippert MM, O'Toole PS, Thorburn A (2006) Autophagy in cancer: good, bad, or both? Cancer Res 66(19):9349–9351. https://doi.org/10.1158/0008-5472.CAN-06-1597
Fulda S, Kogel D (2015) Cell death by autophagy: emerging molecular mechanisms and implications for cancer therapy. Oncogene 34(40):5105–5113. https://doi.org/10.1038/onc.2014.458
Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, Tan KS, Heitjan DF, DeMichele AM, Vaughn DJ, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O'Dwyer PJ, Amaravadi RK (2014) Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy 10(8):1391–1402. https://doi.org/10.4161/auto.29119
Li J, Hou N, Faried A, Tsutsumi S, Kuwano H (2010) Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer 46(10):1900–1909. https://doi.org/10.1016/j.ejca.2010.02.021
Vyas AR, Hahm ER, Arlotti JA, Watkins S, Stolz DB, Desai D, Amin S, Singh SV (2013) Chemoprevention of prostate cancer by d, l-sulforaphane is augmented by pharmacological inhibition of autophagy. Cancer Res 73(19):5985–5995. https://doi.org/10.1158/0008-5472.CAN-13-0755
Herman-Antosiewicz A, Johnson DE, Singh SV (2006) Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res 66(11):5828–5835. https://doi.org/10.1158/0008-5472.CAN-06-0139
Gewirtz DA (2014) The four faces of autophagy: implications for cancer therapy. Cancer Res 74(3):647–651. https://doi.org/10.1158/0008-5472.CAN-13-2966
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, Wang X, He C, Pan H (2013) Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4:e838. https://doi.org/10.1038/cddis.2013.350
Yang ZJ, Chee CE, Huang S, Sinicrope FA (2011) The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 10(9):1533–1541. https://doi.org/10.1158/1535-7163.mct-11-0047
Kumar D, Shankar S, Srivastava RK (2014) Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett 343(2):179–189. https://doi.org/10.1016/j.canlet.2013.10.003
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K, Imoto M, Hattori N (2011) Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 7(2):176–187. https://doi.org/10.4161/auto.7.2.14074
Farrow JM, Yang JC, Evans CP (2014) Autophagy as a modulator and target in prostate cancer. Nat Rev Urol 11(9):508–516. https://doi.org/10.1038/nrurol.2014.196
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, Guo J, Chen X, Wang Y (2011) Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med 6(1):27. https://doi.org/10.1186/1749-8546-6-27
Song M, Liu X, Liu K, Zhao R, Huang H, Shi Y, Zhang M, Zhou S, Xie H, Chen H, Li Y, Zheng Y, Wu Q, Liu F, Li E, Bode AM, Dong Z, Lee MH (2018) Targeting AKT with oridonin inhibits growth of esophageal squamous cell carcinoma in vitro and patient-derived xenografts in vivo. Mol Cancer Ther 17(7):1540–1553. https://doi.org/10.1158/1535-7163.mct-17-0823
Li Y, Wang Y, Wang S, Gao Y, Zhang X, Lu C (2015) Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med Oncol 32(1):365. https://doi.org/10.1007/s12032-014-0365-1
Ding Y, Ding C, Ye N, Liu Z, Wold EA, Chen H, Wild C, Shen Q, Zhou J (2016) Discovery and development of natural product oridonin-inspired anticancer agents. Eur J Med Chem 122:102–117. https://doi.org/10.1016/j.ejmech.2016.06.015
Liu H, Zhu W, Zhu C, Wang Q, Ke Y, Liu Z, Yan X, Zhang J, Qu H Novel ent-kaurene diterpene compound and its derivatives, their preparation and their use. Patent No. 8084430 (in the United States).
Liu H, Zhu W, Zhu C, Wang Q, Ke Y, Liu Z, Yan X, Zhang J, H Q Novel ent-kaurene intervene compound and its derivatives, their preparation and their use. Patent No. AZ (in China).
Peng KZ, Ke Y, Zhao Q, Tian F, Liu HM, Hou G, Lu Z (2017) OP16, a novel ent-kaurene diterpenoid, potentiates the antitumor effect of rapamycin by inhibiting rapamycin-induced feedback activation of Akt signaling in esophageal squamous cell carcinoma. Biochem Pharmacol 140:16–27. https://doi.org/10.1016/j.bcp.2017.05.013
Hou G, Zhang Q, Wang L, Liu M, Wang J, Xue L (2010) mTOR inhibitor rapamycin alone or combined with cisplatin inhibits growth of esophageal squamous cell carcinoma in nude mice. Cancer Lett 290(2):248–254. https://doi.org/10.1016/j.canlet.2009.09.015
Hou G, Zhao Q, Zhang M, Fan T, Liu M, Shi X, Ren Y, Wang Y, Zhou J, Lu Z (2018) Down-regulation of Rictor enhances cell sensitivity to PI3K inhibitor LY294002 by blocking mTORC2-medicated phosphorylation of Akt/PRAS40 in esophageal squamous cell carcinoma. Biomed Pharmacother 106:1348–1356. https://doi.org/10.1016/j.biopha.2018.07.075
Chen L, Lu D, Sun K, Xu Y, Hu P, Li X, Xu F (2019) Identification of biomarkers associated with diagnosis and prognosis of colorectal cancer patients based on integrated bioinformatics analysis. Gene 692:119–125. https://doi.org/10.1016/j.gene.2019.01.001
von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B (2003) STRING: a database of predicted functional associations between proteins. Nucleic Acids Res 31(1):258–261. https://doi.org/10.1093/nar/gkg034
Liu Z, Ouyang L, Peng H, Zhang WZ (2012) Oridonin: targeting programmed cell death pathways as an anti-tumour agent. Cell Prolif 45(6):499–507. https://doi.org/10.1111/j.1365-2184.2012.00849.x
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141. https://doi.org/10.1038/ncb2152
Sotthibundhu A, McDonagh K, von Kriegsheim A, Garcia-Munoz A, Klawiter A, Thompson K, Chauhan KD, Krawczyk J, McInerney V, Dockery P, Devine MJ, Kunath T, Barry F, O'Brien T, Shen S (2016) Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res Ther 7(1):166. https://doi.org/10.1186/s13287-016-0425-x
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. https://doi.org/10.3322/caac.20107
Chen J, Pan J, Zheng X, Zhu K, Li J, Chen M, Wang J, Liao Z (2012) Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 82(1):475–482. https://doi.org/10.1016/j.ijrobp.2010.08.037
Racanelli AC, Kikkers SA, Choi AMK, Cloonan SM (2018) Autophagy and inflammation in chronic respiratory disease. Autophagy 14(2):221–232. https://doi.org/10.1080/15548627.2017.1389823
Karabiyik C, Lee MJ, Rubinsztein DC (2017) Autophagy impairment in Parkinson's disease. Essays Biochem 61(6):711–720. https://doi.org/10.1042/ebc20170023
Vogl DT, Stadtmauer EA, Tan KS, Heitjan DF, Davis LE, Pontiggia L, Rangwala R, Piao S, Chang YC, Scott EC, Paul TM, Nichols CW, Porter DL, Kaplan J, Mallon G, Bradner JE, Amaravadi RK (2014) Combined autophagy and proteasome inhibition: a phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy 10(8):1380–1390. https://doi.org/10.4161/auto.29264
Rangwala R, Leone R, Chang YC, Fecher LA, Schuchter LM, Kramer A, Tan KS, Heitjan DF, Rodgers G, Gallagher M, Piao S, Troxel AB, Evans TL, DeMichele AM, Nathanson KL, O'Dwyer PJ, Kaiser J, Pontiggia L, Davis LE, Amaravadi RK (2014) Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy 10(8):1369–1379. https://doi.org/10.4161/auto.29118
Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi RK, Davis LE, Mita AC, Curiel TJ, Espitia CM, Nawrocki ST, Giles FJ, Carew JS (2014) Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy 10(8):1403–1414. https://doi.org/10.4161/auto.29231
Hall TM, Tetreault MP, Hamilton KE, Whelan KA (2018) Autophagy as a cytoprotective mechanism in esophageal squamous cell carcinoma. Curr Opin Pharmacol 41:12–19. https://doi.org/10.1016/j.coph.2018.04.003
Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, White E (2014) Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov 4(8):914–927. https://doi.org/10.1158/2159-8290.cd-14-0363
Mauvezin C, Neufeld TP (2015) Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 11(8):1437–1438. https://doi.org/10.1080/15548627.2015.1066957
Redmann M, Benavides GA, Berryhill TF, Wani WY, Ouyang X, Johnson MS, Ravi S, Barnes S, Darley-Usmar VM, Zhang J (2017) Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol 11:73–81. https://doi.org/10.1016/j.redox.2016.11.004
Schreiber KH, Arriola Apelo SI, Yu D, Brinkman JA, Velarde MC, Syed FA, Liao CY, Baar EL, Carbajal KA, Sherman DS, Ortiz D, Brunauer R, Yang SE, Tzannis ST, Kennedy BK, Lamming DW (2019) A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun 10(1):3194. https://doi.org/10.1038/s41467-019-11174-0
Luo T, Fu J, Xu A, Su B, Ren Y, Li N, Zhu J, Zhao X, Dai R, Cao J, Wang B, Qin W, Jiang J, Li J, Wu M, Feng G, Chen Y, Wang H (2016) PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy 12(8):1355–1371. https://doi.org/10.1080/15548627.2015.1034405
Liu F, Chen J, Wang P, Li H, Zhou Y, Liu H, Liu Z, Zheng R, Wang L, Yang H, Cui Z, Wang F, Huang X, Wang J, Sha W, Xiao H, Ge B (2018) MicroRNA-27a controls the intracellular survival of Mycobacterium tuberculosis by regulating calcium-associated autophagy. Nat Commun 9(1):4295. https://doi.org/10.1038/s41467-018-06836-4
Jin S, Shen JN, Wang J, Huang G, Zhou JG (2007) Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol Ther 6(2):261–268. https://doi.org/10.4161/cbt.6.2.3621
Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM, Ren Z, Li XM, Shen HM, Zeng HT (2007) Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol Sin 28(11):1819–1826. https://doi.org/10.1111/j.1745-7254.2007.00667.x
Munson MJ, Ganley IG (2015) MTOR, PIK3C3, and autophagy: Signaling the beginning from the end. Autophagy 11(12):2375–2376. https://doi.org/10.1080/15548627.2015.1106668
Gao L, Wang Z, Lu D, Huang J, Liu J, Hong L (2019) Paeonol induces cytoprotective autophagy via blocking the Akt/mTOR pathway in ovarian cancer cells. Cell Death Dis 10(8):609. https://doi.org/10.1038/s41419-019-1849-x
Sun Y, Huang YH, Huang FY, Mei WL, Liu Q, Wang CC, Lin YY, Huang C, Li YN, Dai HF, Tan GH (2018) 3'-epi-12beta-hydroxyfroside, a new cardenolide, induces cytoprotective autophagy via blocking the Hsp90/Akt/mTOR axis in lung cancer cells. Theranostics 8(7):2044–2060. https://doi.org/10.7150/thno.23304
Brenner D, Mak TW (2009) Mitochondrial cell death effectors. Curr Opin Cell Biol 21(6):871–877. https://doi.org/10.1016/j.ceb.2009.09.004
Mundi PS, Sachdev J, McCourt C, Kalinsky K (2016) AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol 82(4):943–956. https://doi.org/10.1111/bcp.13021
Funding
This work was supported by Key Research Project of University, Department of Education of Henan Province (Grant No. 20A350019), Henan Provincial University Science and Technology Innovation Team, Department of Education of Henan Province (Grant No. 19IRTSTHN001) and Key Project of Science and Technology, Department of Science and Technology of Henan Province (Grant No. 202102310127).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest are disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hou, G., Jia, A., Yang, L. et al. OP16 induces deadly autophagy and apoptosis of cells by inhibiting Akt in esophageal squamous cell carcinoma. Mol Cell Biochem 472, 219–230 (2020). https://doi.org/10.1007/s11010-020-03800-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03800-z