Abstract
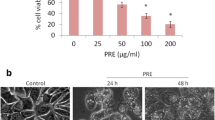
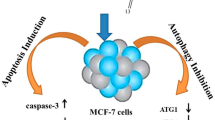
Oridonin is an active diterpenoid, which was extracted from traditional Chinese herbs and had been widely used in clinical treatment nowadays. Oridonin phosphate is one of the derivatives of oridonin. In the present study, we explored its anti-tumor effect and investigated the molecular mechanism of oridonin phosphate in breast cancer cell lines. Firstly, cell viability was analyzed by MTT assay. The breast cancer cells were treated with increasing concentrations of oridonin phosphate for 24, 48 and 72 h, respectively. The results demonstrated that oridonin phosphate inhibited the proliferation of MDA-MB-436 and MDA-MB-231 cells in a dose- and time-dependent manner. Next, cell apoptosis rate was detected in oridonin phosphate-treated breast cancer cells by Annexin V-FITC/PI dual staining analysis and the data demonstrated that oridonin phosphate induced cell apoptosis of breast cancer cells in time- and dose-dependent manner. Moreover, apoptosis-related proteins were detected by Western blotting analysis. The results showed that the expression level of Bax was up-regulated and the expression level of Bcl-2 was down-regulated. Meanwhile, the level of cleaved caspase-9 was significantly increased when the cells were treated with 40 μM of oridonin phosphate for 48 h, although the expression level of pro-caspase-9 was not obviously changed. All of the data revealed that mitochondrial apoptosis pathway may be involved in the cell apoptosis induced by oridonin phosphate in breast cancer cells. Importantly, the expression levels of autophagy-related protein beclin-1 and LC3-II were significantly higher in oridonin phosphate-treated breast cancer cell lines MDA-MB-436 and MDA-MB-231 for 48 h. Additionally, we further explored the relationship between apoptosis and autophagy specifically induced by oridonin phosphate in breast cancer cells. The result showed that inhibition of autophagy suppressed the cell apoptosis in oridonin phosphate-treated MDA-MB-436 cells. Taken together, the compound of oridonin phosphate simultaneously induced cell apoptosis and autophagy in breast cancer cells. Inhibition oridonin phosphate-induced cell autophagy suppressed the progression of cell apoptosis, which revealed that oridonin phosphate-induced autophagy participated in up-regulation of apoptosis in human breast cancer cells. It would provide some new clues for the therapy of breast cancer.







Similar content being viewed by others
References
Battaglini CL, Mills RC, Phillips BL, et al. Twenty-five years of research on the effects of exercise training in breast cancer survivors: a systematic review of the literature. World J Clin Oncol. 2014;5:177–90.
Hiller D, Chu QD. CXCR4 and axillary lymph nodes: review of a potential biomarker for breast cancer metastasis. Int J Breast Cancer. 2011;2011:420981.
Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011;11:384.
Agrawal A, Ayantunde AA, Rampaul R, Robertson JF. Male breast cancer: a review of clinical management. Breast Cancer Res Treat. 2007;103:11–21.
Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–39.
Yang L, Parkin DM, Li L, Chen Y. Time trends in cancer mortality in China: 1987–1999. Int J Cancer. 2003;106:771–83.
Yang L, Li LD, Chen YD, Parkin DM. Time trends, estimates and projects for breast cancer incidence and mortality in China. Zhonghua Zhong Liu Za Zhi. 2006;28:438–40.
Yu X, Deng Q, Bode AM, Dong Z, Cao Y. The role of necroptosis, an alternative form of cell death, in cancer therapy. Expert Rev Anticancer Ther. 2013;13:883–93.
Qi R, Liu XY. New advance in caspase-independent programmed cell death and its potential in cancer therapy. Int J Biomed Sci. 2006;2:211–6.
Maddika S, Ande SR, Panigrahi S, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29.
Yoo JO, Ha KS. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int Rev Cell Mol Biol. 2012;295:139–74.
Liu T, Wu LY, Choi JK, Berkman CE. Targeted photodynamic therapy for prostate cancer: inducing apoptosis via activation of the caspase-8/-3 cascade pathway. Int J Oncol. 2010;36:777–84.
Griffin RJ, Williams BW, Bischof JC, Olin M, Johnson GL, Lee BW. Use of a fluorescently labeled poly-caspase inhibitor for in vivo detection of apoptosis related to vascular-targeting agent arsenic trioxide for cancer therapy. Technol Cancer Res Treat. 2007;6:651–4.
Saha T. LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy. 2012;8:1643–56.
Du XX, Li YJ, Wu CL, et al. Initiation of apoptosis, cell cycle arrest and autophagy of esophageal cancer cells by dihydroartemisinin. Biomed Pharmacother. 2013;67:417–24.
Wang Y, Ding L, Wang X, et al. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am J Transl Res. 2012;4:44–51.
Zeng Y, Li S, Wu J, et al. Autophagy inhibitors promoted aristolochic acid I induced renal tubular epithelial cell apoptosis via mitochondrial pathway but alleviated nonapoptotic cell death in mouse acute aritolochic acid nephropathy model. Apoptosis. 2014;19:1215–24.
Zhao X, Gao S, Ren H, Huang H, Ji W, Hao J. Inhibition of autophagy strengthens celastrol-induced apoptosis in human pancreatic cancer in vitro and in vivo models. Curr Mol Med. 2014;14:555–63.
Fujita E, Fujita T, Ito N. Studies on the constituents of the stems of Isodon trichocarpus Kudo. Yakugaku Zasshi. 1967;87:1150–3.
Fujita E, Nagao Y, Node M, Kaneko K, Nakazawa S, Kuroda H. Antitumor activity of the Isodon diterpenoids: structural requirements for the activity. Experientia. 1976;32:203–6.
Tian W, Chen SY. Recent advances in the molecular basis of anti-neoplastic mechanisms of oridonin. Chin J Integr Med. 2013;19:315–20.
Bao R, Shu Y, Wu X, et al. Oridonin induces apoptosis and cell cycle arrest of gallbladder cancer cells via the mitochondrial pathway. BMC Cancer. 2014;14:217.
Sun KW, Ma YY, Guan TP, et al. Oridonin induces apoptosis in gastric cancer through Apaf-1, cytochrome c and caspase-3 signaling pathway. World J Gastroenterol. 2012;18:7166–74.
Ye LH, Li WJ, Jiang XQ, et al. Study on the autophagy of prostate cancer PC-3 cells induced by oridonin. Anat Rec (Hoboken). 2012;295:417–22.
D’Anneo A, Carlisi D, Lauricella M, et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013;4:e891.
Huang AC, Lien JC, Lin MW, et al. Tetrandrine induces cell death in SAS human oral cancer cells through caspase activation-dependent apoptosis and LC3-I and LC3-II activation-dependent autophagy. Int J Oncol. 2013;43:485–94.
Nishitani H, Sugimoto N, Roukos V, et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–36.
Peng R, Lin J, Wei D. Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa CS-2. Appl Biochem Biotechnol. 2010;162:733–43.
Acknowledgments
This research was supported by Grants from the National Natural Science Foundation of China (No. 81360324) and the Natural Science Foundation of Guangxi Province (No. 2013GXNSFAA019222).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Wang, Y., Wang, S. et al. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med Oncol 32, 365 (2015). https://doi.org/10.1007/s12032-014-0365-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0365-1