Abstract
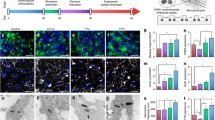
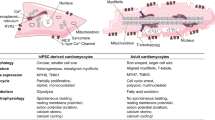
Logistic complexities of heart transplantation embossed the necessity of utilizing novel methods, which enable heart regeneration. Human cardiosphere-derived cells (hCDCs) are taken into consideration as a promising cell resource in cell therapy in recent years. In this study, we designed an electrochemical stimulation system, which sends square pulses to the hCDCs and records their electrical response. Morphology, viability and differentiation of hCDCs are monitored at certain time courses of the treatment. Differentiating hCDCs aligned perpendicularly with respect to the direction of applied electric current, and obtained a spindle-like morphology, while they remained viable. At the same time, specific cardiac marker genes including GATA4, cTnT and α-MHC showed a considerable up-regulation. Our findings confirm that hCDCs differentiate to committed cardiomyocytes when hCDCs receive an electrical energy of 0.06 – 0.12 Wh. This amount of electrical energy could be applied to the stem cells using versatile electrical stimulation patterns via commercially available devices.





Similar content being viewed by others
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R (2018) Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137:e67
Murohara T (2020) Therapeutic Angiogenesis with Somatic Stem Cell Transplantation. Korean Circulation Journal 50:12–21
Miller LW (2019) Limitations of current medical therapies for the treatment of heart failure. Reviews in cardiovascular medicine 4:21–29
Traverse JH, Henry TD, Dib N, Patel AN, Pepine C, Schaer GL, DeQuach JA, Kinsey AM, Chamberlin P, Christman KL (2019) First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC: Basic Translational Science 4:659–669
Menasché P (2015) Stem cells for the treatment of heart failure. Philoso Transac Royal Soc B: Biol Sci 370:20140373
Berger H-J, Prasad SK, Davidoff A, Pimental D, Ellingsen O, Marsh J, Smith T, Kelly R (1994) Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture. Am J Physiol-Heart Circulatory Physiol 266:H341–H349
Wang Y-G, Kim HW and Ma R (2018) Methods for accelerated and enhanced cardiac differentiation of IPS cells by electrical stimulation. Google Patents,
Korolj A, Wang EY, Civitarese RA, Radisic M (2017) Biophysical stimulation for in vitro engineering of functional cardiac tissues. Clin Sci 131:1393–1404
Au HTH, Cheng I, Chowdhury MF, Radisic M (2007) Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials 28:4277–4293
Liew A, André FM, Lesueur LL, De Ménorval M-A, O'Brien T, Mir LM (2013) Robust, efficient, and practical electrogene transfer method for human mesenchymal stem cells using square electric pulses. Human gene therapy methods 24:289–297
Genovese JA, Spadaccio C, Chachques E, Schussler O, Carpentier A, Chachques JC, Patel AN (2009) Cardiac pre-differentiation of human mesenchymal stem cells by electrostimulation. Front Biosci 14:2996–3002
Li T-S, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H (2012) Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 59:942–953
Doppler SA, Deutsch M-A, Lange R, Krane M (2013) Cardiac regeneration: current therapies—future concepts. Journal of thoracic disease 5:683
Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR and Marbán E (2007) Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation.
Jafarzadeh M, Soltani BM, Tousi SE, Behmanesh M (2018) Hsa-miR-497 as a new regulator in TGFβ signaling pathway and cardiac differentiation process. Gene 675:150–156
Matsa E, Burridge PW, Wu JC (2014) Human stem cells for modeling heart disease and for drug discovery. Science translational medicine 6:239ps6–239ps6
Tiryaki VM, Adia-Nimuwa U, Ayres VM, Ahmed I, Shreiber DI (2015) Texture-based segmentation and a new cell shape index for quantitative analysis of cell spreading in AFM images. Cytometry Part A 87:1090–1100
Matsutani S, Yamamoto N (1997) Neuronal regulation of astrocyte morphology in vitro is mediated by GABAergic signaling. Glia 20:1–9
Vartanian KB, Kirkpatrick SJ, Hanson SR, Hinds MT (2008) Endothelial cell cytoskeletal alignment independent of fluid shear stress on micropatterned surfaces. Biochem Biophys Res Commun 371:787–792
Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE (1998) Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog 14:356–363
Tiryaki VM, Ayres VM, Ahmed I, Shreiber DI (2015) Differentiation of reactive-like astrocytes cultured on nanofibrillar and comparative culture surfaces. Nanomedicine 10:529–545
Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park S-Y, Silberstein LE, Dos Remedios CG, Graham D, Colan S (2013) Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci 110:1446–1451
Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE (1998) Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 4:929
Pedrotty DM, Koh J, Davis BH, Taylor DA, Wolf P, Niklason LE (2005) Engineering skeletal myoblasts: roles of three-dimensional culture and electrical stimulation. American Journal of Physiology-Heart and Circulatory Physiology 288:H1620–H1626
Sharma S, Jackson P and Makan J (2004) Cardiac troponins. BMJ Publishing Group,
Ling-Ling E, Zhao Y-S, Guo X-M, Wang C-Y, Jiang H, Li J, Duan C-M, Song Y (2006) Enrichment of cardiomyocytes derived from mouse embryonic stem cells. The Journal of heart and lung transplantation 25:664–674
Morkin E (2000) Control of cardiac myosin heavy chain gene expression. Microsc Res Tech 50:522–531
Mahdavi V, Chambers AP, Nadal-Ginard B (1984) Cardiac alpha-and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci 81:2626–2630
Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci 100:12313–12318
Molkentin JD, Lin Q, Duncan SA, Olson EN (1997) Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev 11:1061–1072
Faustino RS, Behfar A, Perez-Terzic C, Terzic A (2008) Genomic chart guiding embryonic stem cell cardiopoiesis. Genome Biol 9:R6
Yilbas A, Hamilton A, Wang Y, Mach H, Lacroix N, Davis DR, Chen J, Li Q (2014) Activation of GATA4 gene expression at the early stage of cardiac specification. Frontiers in chemistry 2:12
Kim Y, Ma A-G, Kitta K, Fitch SN, Ikeda T, Ihara Y, Simon AR, Evans T, Suzuki YJ (2003) Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol 63:368–377
Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, Rajagopal S, Son JK, Ma Q, Springer Z (2006) Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci 103:14471–14476
Zhao R, Watt AJ, Battle MA, Li J, Bondow BJ, Duncan SA (2008) Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Developmental biology 317:614–619
Yan L, Jia Z, Cui J, Yang H, Yang H, Zhang Y, Zhou C (2011) Beta-adrenergic signals regulate cardiac differentiation of mouse embryonic stem cells via mitogen-activated protein kinase pathways. Dev Growth Differ 53:772–779
Acknowledgements
We wish to thank Iran University of Medical Sciences for the use of their laboratory facilities.
Author information
Authors and Affiliations
Contributions
H.N., I.R. and B.A. conceived this study. H.N. and M.K. performed and analyzed biological experiments and wrote the first draft of manuscript. I.R. performed electrical conductivity experiments and also contributed to the writing of the manuscript. B.A. and MT.J. provided the financial support, designed and analyzed the experiments and discussed the results and approved the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazari, H., Kehtari, M., Rad, I. et al. Electrical stimulation induces differentiation of human cardiosphere-derived cells (hCDCs) to committed cardiomyocyte. Mol Cell Biochem 470, 29–39 (2020). https://doi.org/10.1007/s11010-020-03742-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03742-6