Abstract
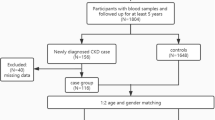
Chronic kidney disease (CKD) is one of the main causes of early death in humans worldwide. Glutathione S-Transferases (GSTs) are involved in a series of xenobiotics metabolism and free radical scavenging. The previous studies elucidated the interlink between GST variants and to the development of various diseases. The present case–control study performed to ascertain whether GST polymorphisms are associated with the incidence and advancement of CKD. From the Southern part of India, a total of 392 CKD patients (nondialysis, ND; n = 170, end-stage renal disease, ESRD; n = 222) and 202 healthy individuals were enrolled. Patients were followed-up for 70 months. Serum biochemical parameters were recorded, and the extraction of DNA was done from the patient’s blood samples. To genotype study participants, multiplex PCR for GSTM1/T1 was performed. Statistical analysis was carried out to analyze the relationship between gene frequency and sonographic grading, as well as biochemical parameters for disease development. The GSTM1-null genotype showed threefold increased risk (OR = 2.9304; 95% CI 1.8959 to 4.5296; P < 0.0001) to CKD development and twofold increased risk (OR = 1.8379; 95% CI 1.1937 to 2.8299; P = 0.0057) to ESRD progression. During the mean follow-up of 41 months study, multivariate Cox regression analysis revealed that GSTM1-null genotype has 4 times increased the risk for all-cause rapid disease progression to ESRD among ND patients and 3.85-fold increased risk for death among ESRD patients. Survival analysis revealed that patients with GSTM1-present allele showed a significantly diminished risk of mortality compared to patients bearing the GSTM1-null allele among ESRD patients with a hazard ratio of 4.6242 (P < 0.0001). Thus, present data confirm that GSTM1-null genotype increased the risk for all-cause rapid disease progression to ESRD among ND patients. Based on our results, GSTM1-null genotype could be considered as a significant predictor for causing mortality among CKD patients when compared to all other variables.

Similar content being viewed by others
References
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389:1238–1252. https://doi.org/10.1016/S0140-6736(16)32064-5
Burden AC, McNally PG, Feehally J, Walls J (1992) Increased incidence of end-stage renal failure secondary to diabetes mellitus in Asian ethnic groups in the United Kingdom. Diabet Med 9:641–645
Dash SC, Agarwal SK (2006) Incidence of chronic kidney disease in India. Nephrol Dial Transplant 21:232–233. https://doi.org/10.1093/ndt/gfi094
Mather HM, Chaturvedi N, Kehely AM (1998) Comparison of prevalence and risk factors for microalbuminuria in South Asians and Europeans with type 2 diabetes mellitus. Diabet Med 15:672–677
CDC (2016) Center for Disease Control and Prevention. Chronic Kidney Disease Basics.,
Ramprasath T, Selvam GS (2013) Potential impact of genetic variants in Nrf2 regulated antioxidant genes and risk prediction of diabetes and associated cardiac complications. Curr Med Chem 20:4680–4693
Ramprasath T, Vasudevan V, Sasikumar S, Puhari SS, Saso L, Selvam GS (2015) Regression of oxidative stress by targeting eNOS and Nrf2/ARE signaling: a guided drug target for cardiovascular diseases. Curr Top Med Chem 15:857–871
Vaziri ND (2004) Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol 24:469–473
Galle J (2001) Oxidative stress in chronic renal failure. Nephrol Dial Transplant 16:2135–2137
Palleschi S, De Angelis S, Diana L, Rossi B, Papa V, Severini G, Splendiani G (2007) Reliability of oxidative stress biomarkers in hemodialysis patients: a comparative study. Clin Chem Lab Med 45:1211–1218. https://doi.org/10.1515/CCLM.2007.266
Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR (2017) Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev 2017:3081856. https://doi.org/10.1155/2017/3081856
Ramprasath T, Senthil Murugan P, Prabakaran AD, Gomathi P, Rathinavel A, Selvam GS (2011) Potential risk modifications of GSTT1, GSTM1 and GSTP1 (glutathione-S-transferases) variants and their association to CAD in patients with type-2 diabetes. Biochem Biophys Res Commun 407:49–53. https://doi.org/10.1016/j.bbrc.2011.02.097
Ramprasath T, Senthamizharasi M, Vasudevan V, Sasikumar S, Yuvaraj S, Selvam GS (2014) Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J Physiol Biochem 70:407–415. https://doi.org/10.1007/s13105-014-0318-3
Dessi M, Noce A, Dawood KF, Galli F, Taccone-Gallucci M, Fabrini R, Bocedi A, Massoud R, Fucci G, Pastore A, Manca di Villahermosa S, Zingaretti V, Federici G, Ricci G (2012) Erythrocyte glutathione transferase: a potential new biomarker in chronic kidney diseases which correlates with plasma homocysteine. Amino Acids 43:347–354. https://doi.org/10.1007/s00726-011-1085-x
Bolt HM, Thier R (2006) Relevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicology. Curr Drug Metab 7:613–628
Parl FF (2005) Glutathione S-transferase genotypes and cancer risk. Cancer Lett 221:123–129. https://doi.org/10.1016/j.canlet.2004.06.016
Lin YS, Hung SC, Wei YH, Tarng DC (2009) GST M1 polymorphism associates with DNA oxidative damage and mortality among hemodialysis patients. J Am Soc Nephrol 20:405–415. https://doi.org/10.1681/ASN.2008020227
Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, Rahman M, Deysher AE, Zhang YL, Schmid CH, Levey AS (2007) Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 18:2749–2757. https://doi.org/10.1681/ASN.2007020199
Nicholas SB, Kalantar-Zadeh K, Norris KC (2015) Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis 22:6–15. https://doi.org/10.1053/j.ackd.2014.07.002
Plantinga LC (2013) Socio-economic impact in CKD. Nephrol Ther 9:1–7. https://doi.org/10.1016/j.nephro.2012.07.361
Merdzo I, Rutkai I, Tokes T, Sure VN, Katakam PV, Busija DW (2016) The mitochondrial function of the cerebral vasculature in insulin-resistant Zucker obese rats. Am J Physiol Heart Circ Physiol 310:H830–H838. https://doi.org/10.1152/ajpheart.00964.2015
Navarro G, Allard C, Morford JJ, Xu W, Liu S, Molinas AJ, Butcher SM, Fine NH, Blandino-Rosano M, Sure VN, Yu S, Zhang R, Munzberg H, Jacobson DA, Katakam PV, Hodson DJ, Bernal-Mizrachi E, Zsombok A, Mauvais-Jarvis F (2018) Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight. https://doi.org/10.1172/jci.insight.98607
Katakam PV, Gordon AO, Sure VN, Rutkai I, Busija DW (2014) Diversity of mitochondria-dependent dilator mechanisms in vascular smooth muscle of cerebral arteries from normal and insulin-resistant rats. Am J Physiol Heart Circ Physiol 307:H493–503. https://doi.org/10.1152/ajpheart.00091.2014
Ramprasath T, Kumar PH, Puhari SS, Murugan PS, Vasudevan V, Selvam GS (2012) L-Arginine ameliorates cardiac left ventricular oxidative stress by upregulating eNOS and Nrf2 target genes in alloxan-induced hyperglycemic rats. Biochem Biophys Res Commun 428:389–394. https://doi.org/10.1016/j.bbrc.2012.10.064
Ramprasath T, Freddy AJ, Velmurugan G, Tomar D, Rekha B, Suvekbala V, Ramasamy S (2020) Context-dependent regulation of nrf2/are axis on vascular cell function during hyperglycemic condition. Curr Diabetes Rev. https://doi.org/10.2174/1573399816666200130094512
Sure VN, Sakamuri S, Sperling JA, Evans WR, Merdzo I, Mostany R, Murfee WL, Busija DW, Katakam PVG (2018) A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience 40:365–375. https://doi.org/10.1007/s11357-018-0037-8
Merdzo I, Rutkai I, Sure V, Katakam PVG, Busija DW (2019) Effects of prolonged type 2 diabetes on mitochondrial function in cerebral blood vessels. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.00341.2019
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. https://doi.org/10.3390/molecules24081583
Poulianiti KP, Kaltsatou A, Mitrou GI, Jamurtas AZ, Koutedakis Y, Maridaki M, Stefanidis I, Sakkas GK, Karatzaferi C (2016) Systemic redox imbalance in chronic kidney disease: a systematic review. Oxid Med Cell Longev 2016:8598253. https://doi.org/10.1155/2016/8598253
Ruiz S, Pergola PE, Zager RA, Vaziri ND (2013) Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83:1029–1041. https://doi.org/10.1038/ki.2012.439
Sung CC, Hsu YC, Chen CC, Lin YF, Wu CC (2013) Oxidative stress and nucleic acid oxidation in patients with chronic kidney disease. Oxid Med Cell Longev 2013:301982. https://doi.org/10.1155/2013/301982
Testa A, Leonardis D, Spoto B, Sanguedolce MC, Parlongo RM, Pisano A, Tripepi G, Mallamaci F, Zoccali C (2016) A polymorphism in a major antioxidant gene (Kelch-like ECH-associated protein 1) predicts incident cardiovascular events in chronic kidney disease patients: an exploratory study. J Hypertens 34:928–934. https://doi.org/10.1097/HJH.0000000000000878
Dounousi E, Bouba I, Spoto B, Pappas K, Tripepi G, Georgiou I, Tselepis A, Elisaf M, Tsakiris D, Zoccali C, Siamopoulos K (2016) A genetic biomarker of oxidative stress, the paraoxonase-1 Q192R gene variant, associates with cardiomyopathy in ckd: a longitudinal study. Oxid Med Cell Longev 2016:1507270. https://doi.org/10.1155/2016/1507270
Dabhi B, Mistry KN (2015) Oxidative stress and its association with TNF-alpha-308 G/C and IL-1alpha-889 C/T gene polymorphisms in patients with diabetes and diabetic nephropathy. Gene 562:197–202. https://doi.org/10.1016/j.gene.2015.02.069
Suvakov S, Damjanovic T, Pekmezovic T, Jakovljevic J, Savic-Radojevic A, Pljesa-Ercegovac M, Radovanovic S, Simic DV, Pljesa S, Zarkovic M, Mimic-Oka J, Dimkovic N, Simic T (2014) Associations of GSTM1*0 and GSTA1*a genotypes with the risk of cardiovascular death among hemodialyses patients. BMC Nephrol 15:12. https://doi.org/10.1186/1471-2369-15-12
Hussain K, Salah N, Hussain S, Hussain S (2012) Investigate the role of Glutathione S Transferase (GST) polymorphism in development of hypertension in UAE population. Iran Red Crescent Med J 14:479–482
Nomani H, Hagh-Nazari L, Aidy A, Vaisi-Raygani A, Kiani A, Rahimi Z, Bahrehmand F, Shakiba E, Mozaffari HR, Tavilani H, Pourmotabbed T (2016) Association between GSTM1, GSTT1, and GSTP1 variants and the risk of end stage renal disease. Ren Fail 38:1455–1461. https://doi.org/10.1080/0886022X.2016.1214054
Gutierrez-Amavizca BE, Orozco-Castellanos R, Ortiz-Orozco R, Padilla-Gutierrez J, Valle Y, Gutierrez-Gutierrez N, Garcia-Garcia G, Gallegos-Arreola M, Figuera LE (2013) Contribution of GSTM1, GSTT1, and MTHFR polymorphisms to end-stage renal disease of unknown etiology in Mexicans. Indian J Nephrol 23:438–443. https://doi.org/10.4103/0971-4065.120342.shall
Acknowledgements
The research presented in this article was supported by the University Grants Commission (UGC), New Delhi, India, through CEGS, CAS,and UPE schemes. V. Vasudevan thanks UGC for the award of Genomics-Meritorious Research Fellowships.
Author information
Authors and Affiliations
Contributions
Conceptualization—VV, TR, GSS; Methodology and Sample collection—KS, VV; Formal analysis—VV. Investigation—VV, TR, GSS, SSMP, and SY; Writing—Original draft—VV, TR; Review and Editing—VV, TR, and GSS; Funding acquisition—GSS.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Data availability statement
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vasudevan, V., Ramprasath, T., Sampathkumar, K. et al. GSTM1-null allele predicts rapid disease progression in nondialysis patients and mortality among South Indian ESRD patients. Mol Cell Biochem 469, 21–28 (2020). https://doi.org/10.1007/s11010-020-03724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03724-8