Abstract
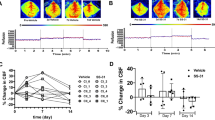
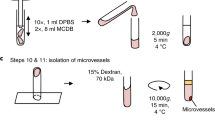
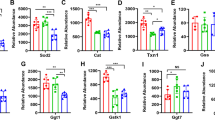
Cerebral blood flow (CBF) is uniquely regulated by the anatomical design of the cerebral vasculature as well as through neurovascular coupling. The process of directing the CBF to meet the energy demands of neuronal activity is referred to as neurovascular coupling. Microvasculature in the brain constitutes the critical component of the neurovascular coupling. Mitochondria provide the majority of ATP to meet the high-energy demand of the brain. Impairment of mitochondrial function plays a central role in several age-related diseases such as hypertension, ischemic brain injury, Alzheimer’s disease, and Parkinson disease. Interestingly, microvessels and small arteries of the brain have been the focus of the studies implicating the vascular mechanisms in several age-related neurological diseases. However, the role of microvascular mitochondrial dysfunction in age-related diseases remains unexplored. To date, high-throughput assay for measuring mitochondrial respiration in microvessels is lacking. The current study presents a novel method to measure mitochondrial respiratory parameters in freshly isolated microvessels from mouse brain ex vivo using Seahorse XFe24 Analyzer. We validated the method by demonstrating impairments of mitochondrial respiration in cerebral microvessels isolated from old mice compared to the young mice. Thus, application of mitochondrial respiration studies in microvessels will help identify novel vascular mechanisms underlying a variety of age-related neurological diseases.




Similar content being viewed by others
References
Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738
Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A, Cash AD, Obrenovich ME, Perry G, Smith MA (2002) Role of mitochondrial dysfunction in Alzheimer’s disease. J Neurosci Res 70:357–360
Chan PH (2005) Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N Y Acad Sci 1042:203–209
Clark LC (1956) Monitor and control of blood and issue oxygen tensions. Trans Am Soc Artif Intern Organs 2:41–48
Clark LC Jr, Sachs G (1968) Bioelectrodes for tissue metabolism. Ann N Y Acad Sci 148:133–153
Csiszar A, Tarantini S, Fulop GA et al (2017) Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience 39:359–372
Dai DF, Rabinovitch PS, Ungvari Z (2012) Mitochondria and cardiovascular aging. Circ Res 110:1109–1124
de la Torre JC (1994) Impaired brain microcirculation may trigger Alzheimer’s disease. Neurosci Biobehav Rev 18:397–401
Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, van Remmen H, Richardson A (2017) A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience 39:187–198
Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci 26:9057–9068
Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64:575–611
Ferrick DA, Neilson A, Beeson C (2008) Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 13:268–274
Girouard H, Iadecola C (2006) Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 100:328–335
Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol (1985) 80:988–998
Grimmig B, Kim SH, Nash K, Bickford PC, Douglas SR (2017) Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience 39:19–32
Horan MP, Pichaud N, Ballard JW (2012) Review: quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A Biol Sci Med Sci 67:1022–1035
Iadecola C (1993) Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci 16:206–214
Izzo C, Carrizzo A, Alfano A, Virtuoso N, Capunzo M, Calabrese M, de Simone E, Sciarretta S, Frati G, Oliveti M, Damato A, Ambrosio M, de Caro F, Remondelli P, Vecchione C (2018) The impact of aging on cardio and cerebrovascular diseases. Int J Mol Sci 19
Jung SK, Gorski W, Aspinwall CA, Kauri LM, Kennedy RT (1999) Oxygen microsensor and its application to single cells and mouse pancreatic islets. Anal Chem 71:3642–3649
Katakam PV, Gordon AO, Sure VN, Rutkai I, Busija DW (2014) Diversity of mitochondria-dependent dilator mechanisms in vascular smooth muscle of cerebral arteries from normal and insulin-resistant rats. Am J Physiol Heart Circ Physiol 307:H493–H503
Katakam PV, Dutta S, Sure VN et al (2016) Depolarization of mitochondria in neurons promotes activation of nitric oxide synthase and generation of nitric oxide. Am J Physiol Heart Circ Physiol 310:H1097–H1106
Kunz A, Iadecola C (2009) Cerebral vascular dysregulation in the ischemic brain. Handb Clin Neurol 92:283–305
Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C (2013) Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol 305:H459–H476
Merdzo I, Rutkai I, Tokes T, Sure VN, Katakam PV, Busija DW (2016) The mitochondrial function of the cerebral vasculature in insulin-resistant Zucker obese rats. Am J Physiol Heart Circ Physiol 310:H830–H838
Merdzo I, Rutkai I, Sure VN, McNulty CA, Katakam PV, Busija DW (2017) Impaired mitochondrial respiration in large cerebral arteries of rats with type 2 diabetes. J Vasc Res 54:1–12
Navarro G, Allard C, Morford JJ, Xu W, Liu S, Molinas AJR, Butcher SM, Fine NHF, Blandino-Rosano M, Sure VN, Yu S, Zhang R, Münzberg H, Jacobson DA, Katakam PV, Hodson DJ, Bernal-Mizrachi E, Zsombok A, Mauvais-Jarvis F (2018) Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 3
Nehlig A (2004) Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fat Acids 70:265–275
Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR (2009) Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58:718–725
Payne BA, Chinnery PF (1847) Mitochondrial dysfunction in aging: much progress but many unresolved questions. Biochim Biophys Acta 2015:1347–1353
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med 14:45–53
Sakamuri S, Sperling JA, Sure VN et al (2018) Measurement of respiratory function in isolated cardiac mitochondria using Seahorse XFe24 Analyzer: applications for aging research. Geroscience 40:347–356
Silbergeld DL, Ali-Osman F (1991) Isolation and characterization of microvessels from normal brain and brain tumors. J Neuro-Oncol 11:49–55
Sonntag WE, Lynch CD, Cooney PT, Hutchins PM (1997) Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology 138:3515–3520
Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z (2015) Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci 70:1355–1359
Starkov AA, Chinopoulos C, Fiskum G (2004) Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium 36:257–264
Stirone C, Duckles SP, Krause DN, Procaccio V (2005) Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68:959–965
Sure VN, Katakam PV (2016) Janus face of thrombospondin-4: impairs small artery vasodilation but protects against cardiac hypertrophy and aortic aneurysm formation. Am J Physiol Heart Circ Physiol 310:H1383–H1384
Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A (2017a) Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience 39:465–473
Tarantini S, Yabluchanksiy A, Fulop GA et al (2017b) Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience 39:601–614
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z (2018) Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17
Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fülöp G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z (2017) Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience 39:385–406
Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A (2010a) Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65:1028–1041
Ungvari Z, Sonntag WE, Csiszar A (2010b) Mitochondria and aging in the vascular system. J Mol Med (Berl) 88:1021–1027
van Hall G, Stromstad M, Rasmussen P et al (2009) Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29:1121–1129
Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Müller MJ (2010) Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 92:1369–1377
Williams LR, Leggett RW (1989) Reference values for resting blood flow to organs of man. Clin Phys Physiol Meas 10:187–217
Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA (2007) Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 292:C125–C136
Yousif S, Marie-Claire C, Roux F, Scherrmann JM, Decleves X (2007) Expression of drug transporters at the blood-brain barrier using an optimized isolated rat brain microvessel strategy. Brain Res 1134:1–11
Yu H, Yang C, Chen S, Huang Y, Liu C, Liu J, Yin W (2017) Comparison of the glycopattern alterations of mitochondrial proteins in cerebral cortex between rat Alzheimer’s disease and the cerebral ischemia model. Sci Rep 7:39948
Zhang J, Nuebel E, Wisidagama DR et al (2012) Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc 7:1068–1085
Acknowledgments
We thank Ms. Sufen Zheng for her technical help for the studies.
Funding
This research project was supported by the National Institutes of Health: National Institute of Neurological Disorders and Stroke and National Institute of General Medical Sciences (NS094834—PV Katakam), National Heart, Lung and Blood Institute (HL-077731 and HL093554—DW Busija), National Institute on Aging (R01AG047296—R Mostany, R01AG049821—WL Murfee); American Heart Association (National Center Scientist Development Grant, 14SDG20490359—PV Katakam, Greatersoutheast Affiliate Predoctoral Fellowship Grant,16PRE27790122—V.N. Sure), Louisiana Board of Regents grants (Endowed Chairs for Eminent Scholars program; DW Busija, RCS, LEQSF(2016-19)-RD-A-24—R Mostany), and COBRE on Aging and Regenerative Medicine (P20GM103629—R Mostany). The content does not necessarily represent the official views of the NIH and is solely the responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental protocols were approved by the Institution of Animal Care and Use Committee (IACUC) of Tulane University School of Medicine in accordance with the National institute of Health (NIH) guidelines for the animal care and use.
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Sure, V.N., Sakamuri, S.S.V.P., Sperling, J.A. et al. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. GeroScience 40, 365–375 (2018). https://doi.org/10.1007/s11357-018-0037-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-018-0037-8