Abstract
Shrm4 is a protein that is exclusively expressed in polarized tissues. The physiological function of Shrm4 in the brain was required to be elucidated. Thus, we aimed to explore how the Shrm4-mediated gamma-aminobutyric acid (GABA) pathway affected neural stem cells (NSCs). At first, the Nestin expression in cultured NSCs was identified. After determination of the interaction of Shrm4 and GABAB1, a series of in vitro experiment were performed to detect cell proliferation, the ability of cell colony formation, degree that NSCs differentiated into neurons, the apoptosis rate, and cell cycle. The levels of Shrm4, GABAB1, Bcl-2-associated protein x (Bax), B cell lymphoma 2 (Bcl-2), cleaved Caspase-3, microtubule-associated protein 2 (MAP-2) as well as suppressor of cytokine signaling 2 (SOCS2) were detected to further assess the role of Shrm4 and GABA pathway in NSCs. Initially, we found that Shrm4 could bind to GABAB1, and overexpression of Shrm4 or activation of GABAB1 increased the number of positive cells, and promoted cell viability, colony formation rate and differentiation of NSCs. After overexpression of Shrm4 or activation of GABAB1, cells in the G1 phase were decreased, while those in the S phase were increased with an inhibited cell apoptosis rate in the NSCs. Besides, the overexpression of Shrm4 or activation of GABAB1 upregulated the levels of Shrm4, GABAB1, Bcl-2, MAP-2 and SOCS2, while downregulated Bax and cleaved Caspase-3 in NSCs. Overall, overexpression of Shrm4 activated GABAB1 to stimulate the proliferation and differentiation of NSCs. Thus, Shrm4 might be considered as a novel target for promoting the proliferation and differentiation of NSCs.







Similar content being viewed by others
Change history
24 June 2020
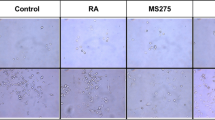
The original publication of the article includes an error in Fig. 2. The correct version of the Fig. 2 is provided in this correction.
References
Shoemaker LD, Kornblum HI (2016) Neural stem cells (NSCs) and proteomics. Mol Cell Proteom 15:344–354
Ottoboni L, Merlini A, Martino G (2017) Neural stem cell plasticity: advantages in therapy for the injured central nervous system. Front Cell Dev Biol 5:52
Yamasaki TR, Blurton-Jones M, Morrissette DA et al (2007) Neural stem cells improve memory in an inducible mouse model of neuronal loss. J Neurosci 27:11925–11933
Chen SQ, Cai Q, Shen YY et al (2014) Combined use of NGF/BDNF/bFGF promotes proliferation and differentiation of neural stem cells in vitro. Int J Dev Neurosci 38:74–78
Wang JH, Hung CH, Young TH (2006) Proliferation and differentiation of neural stem cells on lysine-alanine sequential polymer substrates. Biomaterials 27:3441–3450
Tiwari SK, Agarwal S, Seth B et al (2015) Inhibitory effects of bisphenol-A on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/beta-Catenin pathway. Mol Neurobiol 52:1735–1757
Hagens O, Ballabio A, Kalscheuer V et al (2006) A new standard nomenclature for proteins related to Apx and Shroom. BMC Cell Biol 7:18
Yoder M, Hildebrand JD (2007) Shroom4 (Kiaa1202) is an actin-associated protein implicated in cytoskeletal organization. Cell Motil Cytoskelet 64:49–63
Hagens O, Dubos A, Abidi F et al (2006) Disruptions of the novel KIAA1202 gene are associated with X-linked mental retardation. Hum Genet 118:578–590
Zapata J, Moretto E, Hannan S et al (2017) Epilepsy and intellectual disability linked protein Shrm4 interaction with GABABRs shapes inhibitory neurotransmission. Nat Commun 8:14536
Yoon BE, Lee CJ (2014) GABA as a rising gliotransmitter. Front Neural Circuits 8:141
Cesetti T, Ciccolini F, Li Y (2011) GABA not only a neurotransmitter: osmotic regulation by GABA(A)R signaling. Front Cell Neurosci 6:3
Li Y, Schmidt-Edelkraut U, Poetz F et al (2015) gamma-Aminobutyric A receptor (GABA(A)R) regulates aquaporin 4 expression in the subependymal zone: relevance to neural precursors and water exchange. J Biol Chem 290:4343–4355
Tuo YL, Li XM, Luo J (2015) Long noncoding RNA UCA1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci 19:3403–3411
Yamaguchi M, Seki T, Imayoshi I et al (2016) Neural stem cells and neuro/gliogenesis in the central nervous system: understanding the structural and functional plasticity of the developing, mature, and diseased brain. J Physiol Sci 66:197–206
Rispoli R, Conti C, Celli P et al (2014) Neural stem cells and glioblastoma. Neuroradiol J 27:169–174
Haus DL, Lopez-Velazquez L, Gold EM et al (2016) Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp Neurol 281:1–16
Guo Y, Shi D, Li W et al (2010) Effects of cerebral microvascular endothelial cells and vascular endothelial growth factor on the proliferation and differentiation of NSCs: a comparative study. Br J Neurosurg 24:62–68
Dolka I, Krol M, Sapierzynski R (2016) Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved caspase-3 and p53) expression in canine mammary tumors: an immunohistochemical and prognostic study. Res Vet Sci 105:124–133
Zheng JH, Viacava Follis A, Kriwacki RW et al (2016) Discoveries and controversies in BCL-2 protein-mediated apoptosis. FEBS J 283:2690–2700
Ashkenazi A, Fairbrother WJ, Leverson JD et al (2017) From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16:273–284
Mondal D, Pradhan L, LaRussa VF (2004) Signal transduction pathways involved in the lineage-differentiation of NSCs: can the knowledge gained from blood be used in the brain? Cancer Invest 22:925–943
Gumy LF, Katrukha EA, Grigoriev I et al (2017) MAP2 defines a pre-axonal filtering zone to regulate KIF1-versus KIF5-dependent cargo transport in sensory neurons. Neuron 94(347–362):e347
Scott HJ, Stebbing MJ, Walters CE et al (2006) Differential effects of SOCS2 on neuronal differentiation and morphology. Brain Res 1067:138–145
Acknowledgements
We acknowledge and appreciate our colleagues for their valuable efforts and comments on this paper.
Funding
This study was supported by National Natural Science Foundation of China (No. 81901534).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests associated with the manuscript.
Ethical approval
All protocols for animal experiments were conducted with approval of the Animal Care Committee of The First Hospital of Jilin University, and conformed to the principles and procedures of the Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, R., Guo, K., Wu, B. et al. Overexpression of Shrm4 promotes proliferation and differentiation of neural stem cells through activation of GABA signaling pathway. Mol Cell Biochem 463, 115–126 (2020). https://doi.org/10.1007/s11010-019-03634-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03634-4