Abstract
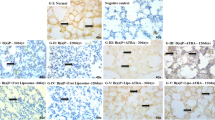
This study aimed to find out the molecular therapeutic effect of lipo-ATRA on tumour suppressor TIG3 and cell proliferative biomarker PPARγ in B (a) P-induced lung cancer model. In RT-PCR study, ATRA- and lipo-ATRA-treated mice samples showed relatively higher TIG3 expression and decreased PPARγ expression (Band density) than cancer control. Among treatments, lipo-ATRA showed vital effect than free ATRA by enhancing TIG3 and decreasing PPARγ. The qPCR results also showed significant (p ≤ 0.05) difference in both TIG3 and PPAR (RQ values of TIG3, lipo-ATRA 23.85 ± 1.29; free ATRA 10.43 ± 1.81 and for PPARγ, lipo-ATRA 4.707 ± 1.21; free ATRA 15.78 ± 2.34). From this, we conclude that liposomal ATRA formulation is most preferable for prolonged delivery of ATRA at targeted site to favour molecular action. It implies that the therapeutic effect of lipo-ATRA in lung cancer was exhibited by ameliorating the TIG3 expression and by suppressing the expression of PPARγ.



Similar content being viewed by others
Abbreviations
- TIG3:
-
Tazarotene-induced gene 3
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- RAR:
-
Retinoic acid receptor
- RXR:
-
Retinoid X receptor
- ATRA:
-
All trans retinoic acid
- RARE:
-
Retinoic acid-responsive elements
- B (a) P:
-
Benzo (a) Pyrene
- DOTAP:
-
1, 2-Dioleoyl-3-trimethylammonium-propane
- RQ:
-
Relative quantity
References
World Health Statistics (2018) Monitoring health for sustainable development goals. World Health Organization 2018
Toh CK (2009) The changing epidemiology of lung cancer. Cancer epidemiology. Humana Press, New York, pp 397–411. https://doi.org/10.1007/978-1-60327-492-0_19
Pleasance ED, Stephens PJ, O’meara S S, McBride DJ, Meynert A, Jones D et al (2010) A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 463(7278):184. https://doi.org/10.1038/nature08629
Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M (2010) Tobacco smoke promotes lung tumorigenesis by triggering IKKβ-and JNK1-dependent inflammation. Cancer Cell 17(1):89–97. https://doi.org/10.1016/j.ccr.2009.12.008
Hecht SS (2012) Lung carcinogenesis by tobacco smoke. Int J Cancer 131(12):2724–2732. https://doi.org/10.1002/ijc.27816
Kim JH, Yamaguchi K, Lee SH, Tithof PK, Sayler GS, Yoon JH, Baek SJ (2005) Evaluation of polycyclic aromatic hydrocarbons in the activation of early growth response-1 and peroxisome proliferator activated receptors. ToxicologicalSciences 85(1):585–593. https://doi.org/10.1093/toxsci/kfi118
Shafey O, Eriksen M, Ross H, Mackay J (2009) The tobacco atlas. Atlanta 3:38–39
Kometani T, Yoshino I, Miura N, Okazaki H, Ohba T, Takenaka T, Maehara Y (2009) Benzo [a] pyrene promotes proliferation of human lung cancer cells by accelerating the epidermal growth factor receptor signaling pathway. Cancer Lett 278(1):27–33. https://doi.org/10.1016/j.canlet.2008.12.017
Klaunig JE, Wang Z, Pu X, Zhou S (2011) Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol 254(2):86–99. https://doi.org/10.1016/j.taap.2009.11.028
Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P (2002) Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 21(48):7435. https://doi.org/10.1038/sj.onc.1205803
Kasala ER, Bodduluru LN, Barua CC, Sriram CS, Gogoi R (2015) Benzo (a) pyrene induced lung cancer: role of dietary phytochemicals in chemoprevention. Pharmacol Rep 67(5):996–1009. https://doi.org/10.1016/j.pharep.2015.03.004
Miller KP, Ramos KS (2001) Impact of cellular metabolism on the biological effects of benzo [a] pyrene and related hydrocarbons. Drug Metab Rev 33(1):1–35. https://doi.org/10.1081/DMR-100000138
Kang L, Gao Z, Huang W, Jin M, Wang Q (2015) Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm Sin B 5(3):169–175. https://doi.org/10.1016/j.apsb.2015.03.001
Barr Kumarakulasinghe N, Zanwijk NV, Soo RA (2015) Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology 20(3):370–378. https://doi.org/10.1111/resp.12490
Regazzi MB, Iacona I, Gervasutti C, Lazzarino M, Toma S (1997) Clinical pharmacokinetics of tretinoin. Clin Pharmacokinet 32(5):382–402. https://doi.org/10.2165/00003088-199732050-00004
Ozpolat B, Lopez-Berestein G, Adamson P, Fu CJ, Williams AH (2003) Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. J Pharm Pharm Sci 6(2):292–301
McGowan SE, Harvey CS, Jackson SK (1995) Retinoids, retinoic acid receptors, and cytoplasmic retinoid binding proteins in perinatal rat lung fibroblasts. Am J Phys 269(4):L463–L472. https://doi.org/10.1152/ajplung.1995.269.4.L463
Choi EJ, Whang YM, Kim SJ, Kim HJ, Kim YH (2007) Combinational treatment with retinoic acid derivatives in non-small cell lung carcinoma in vitro. J Korean Med Sci 22(Suppl):S52–S60. https://doi.org/10.3346/jkms.2007.22.S.S52
Siddikuzzaman GC, Berlin Grace VM (2011) All trans retinoic acid and cancer. Immunopharmacol Immunotoxicol 33(2):241–249
Higuchi E, Chandraratna RA, Hong WK, Lotan R (2003) Induction of TIG3, a putative class II tumor suppressor gene, by retinoic acid in head and neck and lung carcinoma cells and its association with suppression of the transformed phenotype. Oncogene 22(30):4627. https://doi.org/10.1038/sj.onc.1206235
DiSepio D, Ghosn C, Eckert RL, Deucher A, Robinson N, Duvic M, Nagpal S (1998) Identification and characterization of a retinoid-induced class II tumor suppressor/growth regulatory gene. Proc Natl Acad Sci USA 95(25):14811–14815
Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, Roberts RA (2003) PPARα agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol 33(6):655–780. https://doi.org/10.1080/713608372
Michalik L, Desvergne B, Wahli W (2004) Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer 4(1):61. https://doi.org/10.1038/nrc1254
Genini D, Garcia-Escudero R, Carbone GM, Catapano CV (2012) Transcriptional and non-transcriptional functions of PPARβ/δ in Non-small cell lung cancer. PLoS ONE 7(9):e46009. https://doi.org/10.1371/journal.pone.0046009
Mader S, Chen JY, Chen Z, White J, Chambon P, Gronemeyer H (1993) The patterns of binding of RAR, RXR and TR homo-and heterodimers to direct repeats are dictated by the binding specificites of the DNA binding domains. EMBO J 12(13):5029–5041. https://doi.org/10.1002/j.1460-2075.1993.tb06196.x
Inoue K, Kawahito Y, Tsubouchi Y, Yamada R, Kohno M, Hosokawa Y, Sano H (2001) Expression of peroxisome proliferator-activated receptor (PPAR)-gamma in human lung cancer. Anticancer Res 21(4A):2471–2476 (PMID:11724309)
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405(6785):421. https://doi.org/10.1038/35013000
Georgiadi A, Kersten S (2012) Mechanisms of gene regulation by fatty acids. Adv Nutr 3(2):127–134. https://doi.org/10.3945/an.111.001602
Grace VB, Viswanathan S (2017) Pharmacokinetics and therapeutic efficiency of a novel cationic liposome nano-formulated all trans retinoic acid in lung cancer mice model. J Drug Deliv Sci Technol 39:223–236. https://doi.org/10.1016/j.jddst.2017.04.005
Viswanathan S, Grace VB (2018) Reduced RAR-β gene expression in Benzo (a) Pyrene induced lung cancer mice is upregulated by DOTAP lipo-ATRA treatment. Gene 668:18–26. https://doi.org/10.1016/j.gene.2018.05.051
Larsen JE, Minna JD (2011) Molecular biology of lung cancer: clinical implications. Clin Chest Med 32(4):703–740. https://doi.org/10.1016/j.ccm.2011.08.003
Chang WA, Hung JY, Tsai YM, Hsu YL, Chiang HH, Chou SH, Kuo PL (2016) Laricitrin suppresses increased benzo (a) pyrene-induced lung tumor-associated monocyte-derived dendritic cell cancer progression. Oncol Lett 11(3):1783–1790. https://doi.org/10.3892/ol.2016.4153
Chen J, Li Q (2016) Implication of retinoic acid receptor selective signaling in myogenic differentiation. Sci Rep 6:18856. https://doi.org/10.1038/srep18856
Ramya D, Siddikuzzaman, Grace VB (2012) Effect of all-trans retinoic acid (ATRA) on syndecan-1 expression and its chemoprotective effect in benzo (α) pyrene-induced lung cancer mice model. Immunopharmacol Immunotoxicol 34(6):1020–1027. https://doi.org/10.3109/08923973.2012.693086
Huang SL, Shyu RY, Yeh MY, Jiang SY (2000) Cloning and characterization of a novel retinoid-inducible gene 1 (RIG1) deriving from human gastric cancer cells. Mol Cell Endocrinol 159(1–2):15–24. https://doi.org/10.1016/S0303-7207(99)00207-5
Mangelsdorf DJ (1994) Vitamin A receptors. Nutr Rev 52(2):S32–S44
Han S, Roman J (2007) Peroxisome proliferator-activated receptor γ: a novel target for cancer therapeutics? Anticancer Drugs 18(3):237–244. https://doi.org/10.1097/CAD.0b013e328011e67d
Li MY, Yuan H, Ma LT, Kong AW, Hsin MK, Yip JH, Chen GG (2010) Roles of peroxisome proliferator-activated receptor–α and–γ in the Development of non-small cell lung cancer. Am J Respir Cell Mol Biol 43(6):674–683. https://doi.org/10.1165/rcmb.2009-0349OC
Berry DC, Noy N (2009) All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor β/δ and retinoic acid receptor. Mol Cell Biol 29(12):3286–3296. https://doi.org/10.1128/MCB.01742-08
Acknowledgements
We are very grateful to thank the Department of Science and Technology-Science and Engineering Research board (DST-SERB), Govt. of India [SB/YS/LS-252/2013 (May 15, 2014)], Department of Biotechnology (DBT), Govt of India [BT/PR14632/NNT/28/824/2015] and Karunya short-term Research grant 2018–2019 [KITS/KSG/56/2018] for the financial support given to complete this work successfully. We extend our thanks to Ms. Perinba Danisha J, SRF, KITS, and Mr. P. Jeyakumar for their valuable guidance and technical assistance during this research work. We acknowledge the Karunya Institute of Technology and Sciences, Coimbatore, for providing instruments and laboratory facilities. We also thank the timely help rendered by the authorities of Sugarcane Breeding Institute, Coimbatore, to carry out the real-time qPCR for the gene expression study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ravichandran, R., Viswanathan, S., Berlin Grace, V.M. et al. Ameliorating effect of lipo-ATRA treatment on the expression of TIG3 and its suppressing effect on PPARγ gene expression in lung cancer animal model. Mol Cell Biochem 460, 105–112 (2019). https://doi.org/10.1007/s11010-019-03574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03574-z