Abstract
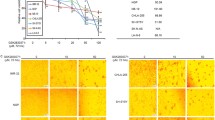
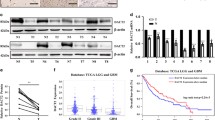
Neuroblastoma is the most common extra-cranial tumor in childhood. As an antineoplastic medicine, the effect of AG-1031 on the neuroblastoma is still unclear. Silent information regulator 1 (SIRT1) is a conserved NAD+-dependent deacetylase, which plays a key role in carcinogenesis through the deacetylation of important regulatory proteins, including p53. The purpose of the present study was to determine whether there was a significant anti-tumor effect of AG-1031 on the human neuroblastoma cells through suppressing SIRT1/p53 pathway. Our study showed that AG1031 treatment resulted in a dose-dependent decrease in human neuroblastoma SH-SY5Y cell viability. The data, obtained from both Western blot assay and Hoechst 33258 staining, further showed that AG1031 exhibited strong anti-tumor activity closely associated with significantly increasing apoptotic indices and enhancing oxidative stress levels. Moreover, AG1031 treatment could down-regulate SIRT1 in a dose-dependent manner and up-regulate p53 acetylation, while overexpression of SIRT1 significantly attenuated the anti-tumor effect of AG1031 in SH-SY5Y cells. AG1031 potently induced SH-SY5Y cells apoptosis through suppressing SIRT1/p53 signaling. These data suggest that AG1031 may be used for therapeutic intervention in neuroblastoma treatment.







Similar content being viewed by others
References
Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3(3):203–216. https://doi.org/10.1038/nrc1014
Mondal T, Juvvuna PK, Kirkeby A, Mitra S, Kosalai ST, Traxler L, Hertwig F, Wernig-Zorc S, Miranda C, Deland L, Volland R, Bartenhagen C, Bartsch D, Bandaru S, Engesser A, Subhash S, Martinsson T, Caren H, Akyurek LM, Kurian L, Kanduri M, Huarte M, Kogner P, Fischer M, Kanduri C (2018) Sense-antisense lncRNA pair encoded by locus 6p22.3 determines neuroblastoma susceptibility via the USP36-CHD7-SOX9 regulatory axis. Cancer Cell 33(3):417–434 e417. https://doi.org/10.1016/j.ccell.2018.01.020
Zhang Q, Ma Y, Cheng YF, Li WJ, Zhang Z, Chen SY (2011) Involvement of reactive oxygen species in 2-methoxyestradiol-induced apoptosis in human neuroblastoma cells. Cancer Lett 313(2):201–210. https://doi.org/10.1016/j.canlet.2011.09.005
Naz H, Tarique M, Khan P, Luqman S, Ahamad S, Islam A, Ahmad F, Hassan MI (2018) Evidence of vanillin binding to CAMKIV explains the anti-cancer mechanism in human hepatic carcinoma and neuroblastoma cells. Mol Cell Biochem 438(1–2):35–45. https://doi.org/10.1007/s11010-017-3111-0
Maris JM, Hogarty MD, Bagatell R, Cohn SL (2007) Neuroblastoma Lancet 369(9579):2106–2120. https://doi.org/10.1016/S0140-6736(07)60983-0
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med 341(16):1165–1173. https://doi.org/10.1056/NEJM199910143411601
Sawada T, Kidowaki T, Sakamoto I, Hashida T, Matsumura T, Nakagawa M, Kusunoki T (1984) Neuroblastoma. Mass screening for early detection and its prognosis. Cancer 53(12):2731–2735
Wang HJ, Tashiro S, Onodera S, Ikejima T (2008) Inhibition of insulin-like growth factor 1 receptor signaling enhanced silibinin-induced activation of death receptor and mitochondrial apoptotic pathways in human breast cancer MCF-7 cells. J Pharmacol Sci 107(3):260–269
Corbi G, Conti V, Scapagnini G, Filippelli A, Ferrara N (2012) Role of sirtuins, calorie restriction and physical activity in aging. Front Biosci 4:768–778
Guo Y, Li P, Gao L, Zhang J, Yang Z, Bledsoe G, Chang E, Chao L, Chao J (2017) Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell 16(4):837–846. https://doi.org/10.1111/acel.12615
Rahman S, Islam R (2011) Mammalian Sirt1: insights on its biological functions. CCS 9:11. https://doi.org/10.1186/1478-811X-9-11
Vaziri H, Dessain SK, Eagon EN, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107(2):149–159. https://doi.org/10.1016/S0092-8674(01)00527-X
Yang YH, Hou HY, Haller EM, Nicosia SV, Bai WL (2005) Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. Embo J 24(5):1021–1032. https://doi.org/10.1038/sj.emboj.7600570
Chen L, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293(5535):1653–1657. https://doi.org/10.1126/science.1062374
Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D (2012) Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 150(3):620–632. https://doi.org/10.1016/j.cell.2012.06.027
Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW (2010) Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1 ALPHA. Mol Cell 38(6):864–878. https://doi.org/10.1016/j.molcel.2010.05.023
Mei Z, Zhang X, Yi J, Huang J, He J, Tao Y (2016) Sirtuins in metabolism, DNA repair and cancer. J Exp Clin Cancer Res 35(1):182. https://doi.org/10.1186/s13046-016-0461-5
Martinez-Pastor B, Mostoslavsky R (2012) Sirtuins, metabolism, and cancer. Front Pharmacol 3:22. https://doi.org/10.3389/fphar.2012.00022
Deng CX (2009) SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci 5(2):147–152
Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X (2010) Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol 30(19):4712–4721. https://doi.org/10.1128/MCB.00657-10
Kong S, Kim SJ, Sandal B, Lee SM, Gao B, Zhang DD, Fang D (2011) The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J Biol Chem 286(19):16967–16975. https://doi.org/10.1074/jbc.M111.218206
Wang Y, Wang H, Ge H, Yang Z (2018) AG-1031 induced autophagic cell death and apoptosis in C6 glioma cells associated with Notch-1 signaling pathway. J Cell Biochem 119(7):5893–5903. https://doi.org/10.1002/jcb.26781
Fu J, Wang H, Gao J, Yu M, Wang R, Yang Z, Zhang T (2017) Rapamycin effectively impedes melamine-induced impairments of cognition and synaptic plasticity in Wistar rats. Mol Neurobiol 54(2):819–832. https://doi.org/10.1007/s12035-016-9687-7
Wang H, Jiang T, Li W, Gao N, Zhang T (2018) Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol Lett 282:100–108. https://doi.org/10.1016/j.toxlet.2017.10.021
Lv Y, Lin S, Peng F (2017) SIRT1 gene polymorphisms and risk of lung cancer. Cancer Manag Res 9:381–386. https://doi.org/10.2147/CMAR.S142677
Cheng Y, Cai L, Jiang P, Wang J, Gao C, Feng H, Wang C, Pan H, Yang Y (2013) SIRT1 inhibition by melatonin exerts antitumor activity in human osteosarcoma cells. Eur J Pharmacol 715(1–3):219–229. https://doi.org/10.1016/j.ejphar.2013.05.017
Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Siddiqui IA, Mukhtar H, Ahmad N (2011) Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res 50(2):140–149. https://doi.org/10.1111/j.1600-079X.2010.00823.x
Suzuki K, Hayashi R, Ichikawa T, Imanishi S, Yamada T, Inomata M, Miwa T, Matsui S, Usui I, Urakaze M, Matsuya Y, Ogawa H, Sakurai H, Saiki I, Tobe K (2012) SRT1720, a SIRT1 activator, promotes tumor cell migration, and lung metastasis of breast cancer in mice. Oncol Rep 27(6):1726–1732. https://doi.org/10.3892/or.2012.1750
Li L, Yuan L, Luo J, Gao J, Guo J, Xie X (2013) MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med 13(2):109–117. https://doi.org/10.1007/s10238-012-0186-5
Ye Z, Fang B, Pan J, Zhang N, Huang J, Xie C, Lou T, Cao Z (2017) miR-138 suppresses the proliferation, metastasis and autophagy of non-small cell lung cancer by targeting Sirt1. Oncol Rep 37(6):3244–3252. https://doi.org/10.3892/or.2017.5619
George J, Ahmad N (2016) Mitochondrial sirtuins in cancer: emerging roles and therapeutic potential. Cancer Res 76(9):2500–2506. https://doi.org/10.1158/0008-5472.CAN-15-2733
Roth M, Chen WY (2014) Sorting out functions of sirtuins in cancer. Oncogene 33(13):1609–1620. https://doi.org/10.1038/onc.2013.120
Zhang Q, Zeng SX, Zhang Y, Zhang Y, Ding D, Ye Q, Meroueh SO, Lu H (2012) A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol Med 4(4):298–312. https://doi.org/10.1002/emmm.201100211
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137(3):413–431. https://doi.org/10.1016/j.cell.2009.04.037
Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, Holyoake TL, Chen W, Bhatia R (2012) Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell 21(2):266–281. https://doi.org/10.1016/j.ccr.2011.12.020
Tang Y, Zhao W, Chen Y, Zhao Y, Gu W (2008) Acetylation is indispensable for p53 activation. Cell 133(4):612–626. https://doi.org/10.1016/j.cell.2008.03.025
Lee JS, Lee HJ, Moon BH, Song SH, Lee MO, Shim SH, Kim HS, Lee MC, Kwon JT, Fornace AJ Jr, Kim SU, Cha HJ (2012) Generation of cancerous neural stem cells forming glial tumor by oncogenic stimulation. Stem Cell Rev 8(2):532–545. https://doi.org/10.1007/s12015-011-9280-4
Hussain A, Mohsin J, Prabhu SA, Begum S, Nusri Qel A, Harish G, Javed E, Khan MA, Sharma C (2013) Sulforaphane inhibits growth of human breast cancer cells and augments the therapeutic index of the chemotherapeutic drug, gemcitabine. APJCP 14(10):5855–5860
Nguyen KC, Willmore WG, Tayabali AF (2013) Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology 306:114–123. https://doi.org/10.1016/j.tox.2013.02.010
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275(5303):1132–1136
Basu A, Haldar S (2003) Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxol- or 2-methoxyestradiol-induced apoptosis. FEBS Lett 538(1–3):41–47
Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391(6666):496–499. https://doi.org/10.1038/35160
Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP (2005) p53 isoforms can regulate p53 transcriptional activity. Genes Dev 19(18):2122–2137. https://doi.org/10.1101/gad.1339905
Schott AF, Apel IJ, Nunez G, Clarke MF (1995) Bcl-XL protects cancer cells from p53-mediated apoptosis. Oncogene 11(7):1389–1394
Chiou SK, Rao L, White E (1994) Bcl-2 blocks p53-dependent apoptosis. Mol Cell Biol 14(4):2556–2563
Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR (2000) p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem 275(10):7337–7342
Ran F, An L, Fan Y, Hang H, Wang S (2016) Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys Rep 2(5):100–105. https://doi.org/10.1007/s41048-016-0029-0
Uzilday B, Turkan I, Ozgur R, Sekmen AH (2014) Strategies of ROS regulation and antioxidant defense during transition from C(3) to C(4) photosynthesis in the genus Flaveria under PEG-induced osmotic stress. J Plant Physiol 171(1):65–75. https://doi.org/10.1016/j.jplph.2013.06.016
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215(2):213–219
Rees JN, Florang VR, Anderson DG, Doorn JA (2007) Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem Res Toxicol 20(10):1536–1542. https://doi.org/10.1021/tx700248y
Satomi A, Murakami S, Hashimoto T, Ishida K, Matsuki M, Sonoda M (1995) Significance of superoxide dismutase (SOD) in human colorectal cancer tissue: correlation with malignant intensity. J Gastroenterol 30(2):177–182
Boojar MM, Goodarzi F (2006) Cytotoxicity and the levels of oxidative stress parameters in WI38 cells following 2 macrocyclic crown ethers treatment. Clin Chim Acta 364(1–2):321–327. https://doi.org/10.1016/j.cca.2005.07.033
Wang H, Gao N, Li Z, Yang Z, Zhang T (2016) Autophagy alleviates melamine-induced cell death in PC12 cells via decreasing ROS level. Mol Neurobiol 53(3):1718–1729. https://doi.org/10.1007/s12035-014-9073-2
Barim-Oz O, Sahin H (2016) Oxidative stress and some biochemical parameters during starvation and refeeding in Astacus leptodactylus (Esch., 1823). Cell Mol Biol 62(13):35–43. https://doi.org/10.14715/cmb/2016.62.13.7
Kovacic P, Osuna JA Jr (2000) Mechanisms of anti-cancer agents: emphasis on oxidative stress and electron transfer. Curr Pharm Des 6(3):277–309
Miyazaki S, Kakutani K, Yurube T, Maeno K, Takada T, Zhang Z, Kurakawa T, Terashima Y, Ito M, Ueha T, Matsushita T, Kuroda R, Kurosaka M, Nishida K (2015) Recombinant human SIRT1 protects against nutrient deprivation-induced mitochondrial apoptosis through autophagy induction in human intervertebral disc nucleus pulposus cells. Arthritis Res Ther 17:253. https://doi.org/10.1186/s13075-015-0763-6
Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192(7):1001–1014
Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B, Pouvesle JM, Pape AL (2012) ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer 130(9):2185–2194. https://doi.org/10.1002/ijc.26252
Yin Y, Terauchi Y, Solomon GG, Aizawa S, Rangarajan PN, Yazaki Y, Kadowaki T, Barrett JC (1998) Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature 391(6668):707–710. https://doi.org/10.1038/35648
Russo T, Zambrano N, Esposito F, Ammendola R, Cimino F, Fiscella M, Jackman J, O’Connor PM, Anderson CW, Appella E (1995) A p53-independent pathway for activation of WAF1/CIP1 expression following oxidative stress. J Biol Chem 270(49):29386–29391
Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isono M, Isshiki K, Uzu T, Kashiwagi A, Koya D (2006) Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic Biol Med 40(12):2175–2182. https://doi.org/10.1016/j.freeradbiomed.2006.02.014
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31771148), National nature science foundation of Tianjin (14JCQNJC10500) and 111 Project (B08011).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: JF, TZ. Performed the experiments and analyzed the data: JF, HZ, YZ. Wrote the manuscript: JF, TZ. Authors approved final version for publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fu, J., Zhang, H., Zhang, Y. et al. AG1031 induces apoptosis through suppressing SIRT1/p53 pathway in human neuroblastoma cells. Mol Cell Biochem 454, 165–175 (2019). https://doi.org/10.1007/s11010-018-3461-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3461-2