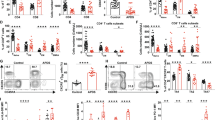
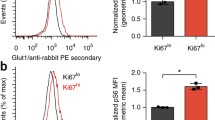
Abstract
Lymphocyte activation is associated with rapid increase of both the glycolytic activator fructose 2,6-bisphosphate (Fru-2,6-P2) and the enzyme responsible for its synthesis, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2/FBPase-2). PFKFB3 gene, which encodes for the most abundant PFK-2 isoenzyme in proliferating tissues, has been found overexpressed during cell activation in several models, including immune cells. However, there is limited knowledge on the pathways underlying PFKFB3 regulation in human T-lymphocytes, and the role of this gene in human immune response. The aim of this work is to elucidate the molecular mechanisms of PFKFB3 induction during human T-lymphocyte activation by mitotic agents. The results obtained showed PFKFB3 induction during human T-lymphocyte activation by mitogens such as phytohemagglutinin (PHA). PFKFB3 increase occurred concomitantly with GLUT-1, HK-II, and PCNA upregulation, showing that mitotic agents induce a metabolic reprograming process that is required for T-cell proliferation. PI3K–Akt pathway inhibitors, Akti-1/2 and LY294002, reduced PFKFB3 gene induction by PHA, as well as Fru-2,6-P2 and lactate production. Moreover, both inhibitors blocked activation and proliferation in response to PHA, showing the importance of PI3K/Akt signaling pathway in the antigen response of T-lymphocytes. These results provide a link between metabolism and T-cell antigen receptor signaling in human lymphocyte biology that can help to better understand the importance of modulating both pathways to target complex diseases involving the activation of the immune system.





Similar content being viewed by others
Abbreviations
- 3PO:
-
3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one
- 7-AAD:
-
7-Aminoactinomycin D
- AICD:
-
Activation-induced cell death
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- ConA:
-
Concanavalin A
- FBS:
-
Fetal bovine serum
- Fru-2,6-P2 :
-
Fructose 2,6-bisphosphate
- GLUT-1:
-
Glucose transporter 1
- HIF:
-
Hypoxia-inducible factor
- HK-II:
-
Hexokinase-II
- IL2RA:
-
Interleukin-2 receptor alpha chain
- LPS:
-
Lipopolysaccharide
- MFI:
-
Mean fluorescence intensity
- mTORC1:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin complex 1
- OXPHOS:
-
Oxidative phosphorylation
- PBMCs:
-
Peripheral blood mononuclear cells
- PCNA:
-
Proliferating cell nuclear antigen
- PFK-1:
-
Phosphofructokinase-1
- PFK-2/FBPase-2:
-
6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase
- PFKFB3:
-
6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase 3
- PHA:
-
Phytohemagglutinin
- P-S6:
-
S6 ribosomal protein
- TCR:
-
T-cell antigen receptor
References
Maciolek JA, Pasternak JA, Wilson HL (2014) Metabolism of activated T lymphocytes. Curr Opin Immunol 27:60–74. https://doi.org/10.1016/j.coi.2014.01.006
Chang CH, Pearce EL (2016) Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol 17(4):364–368. https://doi.org/10.1038/ni.3415
Frauwirth KA, Riley JL, Harris MH et al (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16(6):769–777. https://doi.org/10.1016/S1074-7613(02)00323-0
Marko AJ, Miller RA, Kelman A, Frauwirth KA (2010) Induction of glucose metabolism in stimulated T lymphocytes is regulated by mitogen-activated protein kinase signaling. PLoS ONE 5(11):e15425. https://doi.org/10.1371/journal.pone.0015425
Wang R, Dillon CP, Shi LZ et al (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35(6):871–882. https://doi.org/10.1016/j.immuni.2011.09.021
Colomer D, Vives-Corrons JL, Pujades A, Bartrons R (1987) Control of phosphofructokinase by fructose 2,6-bisphosphate in B-lymphocytes and B-chronic lymphocytic leukemia cells. Cancer Res 47(7):1859–1862
Colomer D, Vives-Corrons JL, Bartrons R (1991) Effect of TPA on fructose 2,6-bisphosphate levels and protein kinase C activity in B-chronic lymphocytic leukemia (B-CLL). Biochim Biophys Acta 1097(4):270–274
Hue L, Rousseau GG (1993) Fructose 2,6-bisphosphate and the control of glycolysis by growth factors, tumor promoters and oncogenes. Adv Enzyme Regul 33:97–110
Telang S, Clem BF, Klarer AC, Clem AL, Trent JO, Bucala R et al (2012) Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J Transl Med 10:95. https://doi.org/10.1186/1479-5876-10-95
Van Schaftingen E (1987) Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol 59:315–395
Okar DA, Manzano A, Navarro-Sabatè A, Riera L, Bartrons R, Lange AJ (2001) PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate. Trends Biochem Sci 26(1):30–35. https://doi.org/10.1016/S0968-0004(00)01699-6
Sakakibara R, Uemura M, Hirata T, Okamura N, Kato M (1997) Human placental fructose-6-phosphate,2-kinase/fructose-2,6-bisphosphatase: its isozymic form, expression and characterization. Biosci Biotechnol Biochem 61(11):1949–1952. https://doi.org/10.1271/bbb.61.1949
Hamilton JA, Callaghan MJ, Sutherland RL, Watts CK (1997) Identification of PRG1, a novel progestin-responsive gene with sequence homology to 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Mol Endocrinol 11(4):490–502. https://doi.org/10.1210/mend.11.4.9909
Manzano A, Rosa JL, Ventura F et al (1998) Molecular cloning, expression, and chromosomal localization of a ubiquitously expressed human 6-phosphofructo-2-kinase/ fructose-2, 6-bisphosphatase gene (PFKFB3). Cytogenet Cell Genet 83(3–4):214–217. https://doi.org/10.1159/000015181
Duran J, Obach M, Navarro-Sabate A et al (2009) Pfkfb3 is transcriptionally upregulated in diabetic mouse liver through proliferative signals. FEBS J 276(16):4555–4568. https://doi.org/10.1111/j.1742-4658.2009.07161.x
Chesney J, Mitchell R, Benigni F et al (1999) An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc Natl Acad Sci USA 96(6):3047–3052. https://doi.org/10.1073/pnas.96.6.3047
Riera L, Manzano A, Navarro-Sabaté A, Perales JC, Bartrons R (2002) Insulin induces PFKFB3 gene expression in HT29 human colon adenocarcinoma cells. Biochim Biophys Acta 1589(2):89–92. https://doi.org/10.1016/S0167-4889(02)00169-6
Calvo MN, Bartrons R, Castaño E, Perales JC, Navarro-Sabaté A, Manzano A (2006) PFKFB3 gene silencing decreases glycolysis, induces cell-cycle delay and inhibits anchorage-independent growth in HeLa cells. FEBS Lett 580(13):3308–3314. https://doi.org/10.1016/j.febslet.2006.04.093
Atsumi T, Chesney J, Metz C et al (2002) High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res 62(20):5881–5887
Kessler R, Bleichert F, Warnke JP, Eschrich K (2008) 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) is up-regulated in high-grade astrocytomas. J Neurooncol 86(3):257–264. https://doi.org/10.1007/s11060-007-9471-7
Obach M, Navarro-Sabaté A, Caro J et al (2004) 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem 279(51):53562–53570. https://doi.org/10.1074/jbc.M406096200
Novellasdemunt L, Obach M, Millán-Ariño L et al (2012) Progestins activate 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) in breast cancer cells. Biochem J 442(2):345–356. https://doi.org/10.1042/BJ20111418
Rodríguez-García A, Samsó P, Fontova P et al (2017) TGF-β1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J 284(20):3437–3454. https://doi.org/10.1111/febs.14201
Ando M, Uehara I, Kogure K et al (2010) Interleukin 6 enhances glycolysis through expression of the glycolytic enzymes hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3. J Nippon Med Sch 77(2):97–105. https://doi.org/10.1272/jnms.77.97
Ruiz-García A, Monsalve E, Novellasdemunt L et al (2011) Cooperation of adenosine with macrophage Toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of PFKFB3 gene. J Biol Chem 286(22):19247–19258. https://doi.org/10.1074/jbc.M110.190298
Novellasdemunt L, Bultot L, Manzano A et al (2013) PFKFB3 activation in cancer cells by the p38/MK2 pathway in response to stress stimuli. Biochem J 452(3):531–543. https://doi.org/10.1042/BJ20121886
Tawakol A, Singh P, Mojena M et al (2015) HIF-1α and PFKFB3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol 35(6):1463–1471. https://doi.org/10.1161/ATVBAHA.115.305551
Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM (2013) Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med 210(10):2119–2134. https://doi.org/10.1084/jem.20130252
Jiang H, Shi H, Sun M et al (2016) PFKFB3-driven macrophage glycolytic metabolism is a crucial component of innate antiviral defense. J Immunol 197(7):2880–2890. https://doi.org/10.4049/jimmunol.1600474
Houddane A, Bultot L, Novellasdemunt L et al (2017) Role of Akt/PKB and PFKFB isoenzymes in the control of glycolysis, cell proliferation and protein synthesis in mitogen-stimulated thymocytes. Cell Signal 34:23–37. https://doi.org/10.1016/j.cellsig.2017.02.019
Lloberas N, Rama I, Llaudó I et al (2013) Dendritic cells phenotype fitting under hypoxia or lipopolysaccharide; adenosine 5′-triphosphate-binding cassette transporters far beyond an efflux pump. Clin Exp Immunol 172(3):444–454. https://doi.org/10.1111/cei.12067
Simon-Molas H, Calvo-Vidal MN, Castaño E et al (2016) Akt mediates TIGAR induction in HeLa cells following PFKFB3 inhibition. FEBS Lett 590(17):2915–2926. https://doi.org/10.1002/1873-3468.12338
Van Schaftingen E, Lederer B, Bartrons R, Hers HG (1982) A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem 129(1):191–195. https://doi.org/10.1111/j.1432-1033.1982.tb07039.x
Gutmann I, Wahlefeld A (1974) L-(+)-lactate determination with lactate dehydrogenase and NAD. In: Bergmeyer H (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York, pp 1464–1468
Nowell PC (1960) Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res 20(4):462–466
Green DR, Droin N, Pinkoski M (2003) Activation-induced cell death in T cells. Immunol Rev 193:70–81. https://doi.org/10.1034/j.1600-065X.2003.00051.x
Wieland E, Shipkova M (2016) Lymphocyte surface molecules as immune activation biomarkers. Clin Biochem 49(4–5):347–354. https://doi.org/10.1016/j.clinbiochem.2015.07.099
Mire-Sluis AR, Wickremasinghe RG, Hoffbrand AV, Timms AM, Francis GE (1987) Human T lymphocytes stimulated by phytohaemagglutinin undergo a single round of cell division without a requirement for interleukin-2 or accessory cells. Immunology 60(1):7–12
Moreno-Aurioles VR, Montaño R, Conde M, Bustos R, Sobrino F (1996) Streptozotocin-induced diabetes increases fructose 2, 6-biphosfhate levels and glucose metabolism in thymus lymphocytes. Life Sci 58(6):477–484. https://doi.org/10.1016/0024-3205(95)02312-7
Chakrabarti R, Jung CY, Lee TP, Liu H, Mookerjee BK (1994) Changes in glucose transport and transporter isoforms during the activation of human peripheral blood lymphocytes by phytohemagglutinin. J Immunol 152(6):2660–2668
Liu C, Chapman NM, Karmaus PW, Zeng H, Chi H (2015) mTOR and metabolic regulation of conventional and regulatory T cells. J Leukoc Biol 97(5):837–847. https://doi.org/10.1189/jlb.2RI0814-408R
Basu S, Hubbard B, Shevach EM (2015) Foxp3-mediated inhibition of Akt inhibits Glut1 (glucose transporter 1) expression in human T regulatory cells. J Leukoc Biol 97(2):279–283. https://doi.org/10.1189/jlb.2AB0514-273RR
Siska PJ, van der Windt GJ, Kishton RJ et al (2016) Suppression of Glut1 and glucose metabolism by decreased Akt/mTORC1 signaling drives T cell impairment in B cell leukemia. J Immunol 197(6):2532–2540. https://doi.org/10.4049/jimmunol.1502464
Trefely S, Khoo PS, Krycer JR et al (2015) Kinome screen identifies PFKFB3 and glucose metabolism as important regulators of the insulin/insulin-like growth factor (IGF)-1 signaling pathway. J Biol Chem 290(43):25834–25846. https://doi.org/10.1074/jbc.M115.658815
Renner K, Geiselhöringer AL, Fante M et al (2015) Metabolic plasticity of human T cells: preserved cytokine production under glucose deprivation or mitochondrial restriction, but 2-deoxy-glucose affects effector functions. Eur J Immunol 45(9):2504–2516. https://doi.org/10.1002/eji.201545473
Nguyen HD, Chatterjee S, Haarberg KM et al (2016) Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest 126(4):1337–1352. https://doi.org/10.1172/JCI82587
De Bock K, Georgiadou M, Schoors S et al (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154(3):651–663. https://doi.org/10.1016/j.cell.2013.06.037
Acknowledgements
We are grateful to B. Torrejón from the Microscopy Unit of Centres Científics i Tecnològics of the Universitat de Barcelona (CCiT-UB) for excellent technical assistance and advice; A. M. Cosialls for technical advice in blood cells cultures; J. L. Rosa and F. Viñals for providing reagents; P. Giménez-Bonafé for English revision and E. Adanero for technical assistance.
Funding
This work was supported by Instituto de Salud Carlos III—FIS [PI13/0096] and FIS [PI17/00412]—and Fondo Europeo de Desarrollo Regional (FEDER), and by Astellas European Foundation Award (13th European Society of Transplantation). HS was recipient of a fellowship from Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya and AR was recipient of a fellowship from the University of Barcelona.
Author information
Authors and Affiliations
Contributions
RB and AM conceived and designed the experiments; HS, CA, PF, and AV performed the experiments; HS, CA, AM, PF, AV, and EC analyzed the data; NLL, AR, and AN critically commented and revised the work; and HS, RB, and AM wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Helga Simon-Molas and Claudia Arnedo-Pac have contributed equally to this work.
Anna Manzano and Ramon Bartrons share senior co-authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simon-Molas, H., Arnedo-Pac, C., Fontova, P. et al. PI3K–Akt signaling controls PFKFB3 expression during human T-lymphocyte activation. Mol Cell Biochem 448, 187–197 (2018). https://doi.org/10.1007/s11010-018-3325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3325-9