Abstract
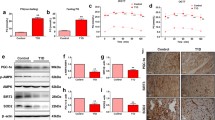
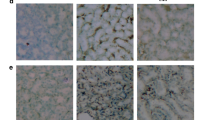
The increased intestinal permeability and functional impairment play an important role in type 2 diabetes (T2D), and melatonin may possess enteroprotection properties. Therefore, we used streptozotocin-induced diabetic rat model to investigate the regulation of intestinal permeability by melatonin. Rats were randomly divided into three groups, including control, diabetes mellitus (DM), and DM rats treated with melatonin. Melatonin was administered (10 mg/kg/day) by gavage for 24 weeks. The DM rats significantly increased the serum fasting blood glucose and lipid levels, which were alleviated by melatonin treatment. Importantly, the intestinal epithelial permeability was significantly increased in DM rats but was ameliorated following treatment with melatonin. These findings also indicated the expression of myosin light chain kinase (MLCK) and phosphorylation of MLC targeting subunit (MYPT) induced myosin light chain (MLC) phosphorylation level was markedly elevated in hyperglycemic and hyperlipidemic status. They were partly associated with down-regulated membrane type 1 and 2 (MT1 and MT2) expression, and up-regulated Rho-associated protein kinase (ROCK) expression and increased extracellular signal-regulated kinase (ERK) phosphorylation. However, the changes in target protein expression were reversed by melatonin. In conclusion, our results show melatonin beneficial effects on impaired intestinal epithelial permeability in T2D by suppressing ERK/MLCK- and ROCK/MCLP-dependent MLC phosphorylation.





Similar content being viewed by others
References
Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M (2002) GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol 97(3):604–611
Visser J, Rozing J, Sapone A, Lammers K, Fasano A (2009) Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci 1165:195–205
Arrieta MC, Bistritz L, Meddings JB (2006) Alterations in intestinal permeability. Gut 55:1512–1520
Mooradian AD, Morley JE, Levine AS, Prigge WF, Gebhard RL (1986) Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia 29:221–224
Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E (2002) Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity 35:365–368
Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A (2006) Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55:1443–1449
Xue Y, Wang H, Du M, Zhu MJ (2014) Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in non-obese diabetic mice. J Nutr Biochem 25:758–764
Galipeau HJ, Rulli NE, Jury J, Huang X, Araya R, Murray JA, David CS, Chirdo FG, McCoy KD, Verdu EF (2011) Sensitization to gliadin induces moderate enteropathy and insulitis in nonobese diabetic-DQ8 mice. J Immunol 187:4338–4346
Horton F, Wright J, Smith L, Hinton PJ, Robertson MD (2014) Increased intestinal permeability to oral chromium (51Cr)-EDTA in human Type 2 diabetes. Diabet Med 31:559–563
Yu M, Yang S, Qiu Y, Chen G, Wang W, Xu C, Cai W, Sun L, Xiao W, Yang H (2015) Par-3 modulates intestinal epithelial barrier function through regulating intracellular trafficking of occludin and myosin light chain phosphorylation. J Gastroenterol 50:1103–1113
Eutamene H, Theodorou V, Schmidlin F, Tondereau V, Garcia-Villar R, Salvador-Cartier C, Chovet M, Bertrand C, Bueno L (2005) LPS-induced lung inflammation is linked to increased epithelial permeability: role of MLCK. Eur Respir J 25:789–796
Al-Sadi R, Ye D, Dokladny K, Ma TY (2008) Mechanism of IL-1beta—induced increase in intestinal epithelial tight junction permeability. J Immunol 180:5653–5661
Suzuki T (2012) Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 70:631–659
Somlyo AP, Somlyo AV (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83:1325–1358
Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR (2005) Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest 115:2702–2715
Chew TL, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL (2002) A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J Cell Biol 156:543–553
Chu J, Miller CT, Kislitsyna K, Laine GA, Stewart RH, Cox CS, Uray KS (2012) Decreased myosin phosphatase target subunit 1(MYPT1) phosphorylation via attenuated rho kinase and zipper-interacting kinase activities in edematous intestinal smooth muscle. Neurogastroenterol Motil 24:257–266
Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ, Zhou Q, Wei W, Zhu HQ, Wang Y (2015) Melatonin attenuates aortic endothelial permeability and arteriosclerosis in streptozotocin-induced diabetic rats: possible role of MLCK- and MLCP-dependent MLC phosphorylation. J Cardiovasc Pharmacol Ther 21:82–92
Ma TY, Boivin MA, Ye D, Pedram A, Said HM (2005) Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol 288:G422–G430
Al-Sadi R, Guo S, Dokladny K, Smith MA, Ye D, Kaza A, Watterson DM, Ma TY (2012) Mechanism of interleukin-1β induced-increase in mouse intestinal permeability in vivo. J Interferon Cytokine Res 32:474–484
Feighery LM, Cochrane SW, Quinn T, Baird AW, O’Toole D, Owens SE, O’Donoghue D, Mrsny RJ, Brayden DJ (2008) Myosin light chain kinase inhibition: correction of increased intestinal epithelial permeability in vitro. Pharm Res 25:1377–1386
Liu X, Xu J, Mei Q, Han L, Huang J (2013) Myosin light chain kinase inhibitor inhibits dextran sulfate sodium-induced colitis in mice. Dig Dis Sci 58:107–114
Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71:2997–3025
Raikhlin NT, Kvetnoy IM, Tolkachev VN (1975) Melatonin may be synthesised in enterochromaffin cells. Nature 255:344–345
Paredes SD, Terrón MP, Marchena AM, Barriga C, Pariente JA, Reiter RJ, Rodríguez AB (2007) Effect of exogenous melatonin on viability, ingestion capacity, and free-radical scavenging in heterophils from young and old ringdoves (Streptopelia risoria). Mol Cell Biochem 304:305–314
Park K, Lee Y, Park S, Lee S, Hong Y, Kil Lee S, Hong Y (2010) Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J Pineal Res 48:270–281
Lee PP, Pang SF (1993) Melatonin and its receptors in the gastrointestinal tract. Biol Signals 2:181–193
Zhu HQ, Cheng XW, Xiao LL, Jiang ZK, Zhou Q, Gui SY, Wei W, Wang Y (2008) Melatonin prevents oxidized low-density lipoprotein-induced increase of myosin light chain kinase activation and expression in HUVEC through ERK/MAPK signal transduction. J Pineal Res 45:328–334
Tan J, Wang Y, Xia Y, Zhang N, Sun X, Yu T, Lin L (2014) Melatonin protects the esophageal epithelial barrier by suppressing the transcription, expression and activity of myosin light chain kinase through ERK1/2 signal transduction. Cell Physiol Biochem 34:2117–2127
Sommansson A, Nylander O, Sjöblom M (2013) Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor–dependent pathway in rats in vivo. J Pineal Res 54:282–291
Lange S, Delbro DS, Jennische E (1994) Evans blue permeation of intestinal mucosa in the rat. Scand J Gastroenterol 29:38–46
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481
Weir GC, Bonner-Weir S (2004) Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53(Suppl 3):S16–S21
Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF (2003) Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetes 52:812–817
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, an risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Yajima S, Morisaki H, Serita R, Suzuki T, Katori N, Asahara T, Nomoto K, Kobayashi F, Ishizaka A, Takeda J (2009) Tumor necrosis factor-alpha mediates hyperglycemia-augmented gut barrier dysfunction in endotoxemia. Crit Care Med 37:1024–1030
Madara JL (1987) Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol 253:C171–C175
Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T et al (1997) Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 272:12257–12260
Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M et al (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245–248
Ruemmele FM, Seidman EG (1998) Cytokine–intestinal epithelial cell interactions: implications for immune mediated bowel disorders. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 39:1–8
Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE (2000) Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355:1518–1519
Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M (2014) Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of pro-inflammation in patients with type 2 diabetes. Mol Cell Biochem 388:203–210
Zhang D, Zhang L, Zheng Y, Yue F, Russell RD, Zeng Y (2014) Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res Clin Pract 106:312–318
Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR (2009) Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136:551–563
Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA (2007) A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One 2:e658
Buendia I, Navarro E, Michalska P, Gameiro I, Egea J, Abril S, López A, González-Lafuente L, López MG, León R (2015) New melatonin-cinnamate hybrids as multi-target drugs for neurodegenerative diseases: Nrf2-induction, antioxidant effect and neuroprotection. Future Med Chem 7:1961–1969
Challet E (2015) Keeping circadian time with hormones. Diabetes Obes Metab 17(Suppl 1):76–83
Sommansson A, Saudi WS, Nylander O, Sjöblom M (2013) Melatonin inhibits alcohol-induced increases in duodenal mucosal permeability in rats in vivo. Am J Physiol Gastrointest Liver Physiol 305:G95–G105
Siah KT, Wong RK, Ho KY (2014) Melatonin for the treatment of irritable bowel syndrome. World J Gastroenterol 20:2492–2498
Lu WZ, Gwee KA, Moochhalla S, Ho KY (2005) Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther 22:927–934
Swanson GR, Gorenz A, Shaikh M, Desai V, Forsyth C, Fogg L, Burgess HJ, Keshavarzian A (2015) Decreased melatonin secretion is associated with increased intestinal permeability and marker of endotoxemia in alcoholics. Am J Physiol Gastrointest Liver Physiol 308:G1004–G1011
Cui P, Yu M, Luo Z, Dai M, Han J, Xiu R, Yang Z (2008) Intracellular signaling pathways involved in cell growth inhibition of human umbilical vein endothelial cells by melatonin. J Pineal Res 44:107–114
Acknowledgments
This study was supported by the National Nature Science Research Grants (Nos.: 81272399, 81470568), Fund of colleges excellent young key projects in Anhui Province (No. 2013SQRL101ZD), and the Anhui Natural Science Foundation (No. 1508085QH167), Doctor fund of Anhui Medical University (No. 0108020103).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Xiaoping Yang, Duobing Zou, and Songtao Tang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, X., Zou, D., Tang, S. et al. Ameliorative effect of melatonin against increased intestinal permeability in diabetic rats: possible involvement of MLCK-dependent MLC phosphorylation. Mol Cell Biochem 416, 23–32 (2016). https://doi.org/10.1007/s11010-016-2691-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2691-4