Abstract
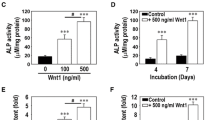
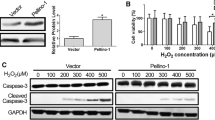
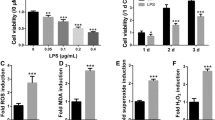
Human periodontal ligament fibroblasts (hPLFs) are exposed to oxidative stress during periodontal inflammation and dental treatments. It is hypothesized that hydrogen peroxide (H2O2)-mediated oxidative stress decreases survival and osteogenic differentiation of hPLFs, whereas these decreases are prevented by activation of the Wnt pathway. However, there has been a lack of reports that define the exact roles of canonical Wnt/β-catenin signaling in H2O2-exposed hPLFs. Treatment with H2O2 reduced viability and proliferation in hPLFs in a dose- and time-dependent manner and led to mitochondria-mediated apoptosis. Pretreatment with lithium chloride (LiCl) or Wnt1 inhibited the oxidative damage that occurred in H2O2-exposed hPLFs. However, knockout of β-catenin or treatment with DKK1 facilitated the H2O2-induced decreases in viability, mitochondrial membrane potential, and Bcl-2 induction. Osteoblastic differentiation of hPLFs was also inhibited by combined treatment with 100 μM H2O2, as evidenced by the decreases in alkaline phosphatase (ALP) activity and mineralization. H2O2-mediated inhibition of osteoblast differentiation in hPLFs was significantly attenuated in the presence of 500 ng/ml Wnt1 or 20 mM LiCl. In particular, H2O2 stimulated the expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) at protein and mRNA levels in hPLFs, whereas the induction was almost completely suppressed in the presence of Wnt1 or LiCl. Furthermore, siRNA-mediated silencing of Nrf2 blocked H2O2-induced decreases in ALP activity and mineralization of hPLFs with the concomitant restoration of runt-related transcription factor 2 and osteocalcin mRNA expression and ALP activity. Collectively, these results suggest that activation of the Wnt/β-catenin pathway improves proliferation and mineralization in H2O2-exposed hPLFs by downregulating Nrf2.







Similar content being viewed by others
References
McCulloch CA, Bordin S (1991) Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res 26:144–154
Roberts WE, Mozsary PG, Klingler E (1982) Nuclear size as a cell-kinetic marker for osteoblast differentiation. Am J Anat 165:373–384
Kook SH, Hwang JM, Park JS, Kim EM, Heo JS, Jeon YM, Lee JC (2009) Mechanical force induces type I collagen expression in human periodontal ligament fibroblasts through activation of ERK/JNK and AP-1. J Cell Biochem 106:1060–1067
Forman HJ, Torres M (2001) Redox signaling in macrophages. Mol Aspects Med 22:189–216
Nordberg J, Arner ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31:1287–1312
Tipton DA, Braxton SD, Dabbous MK (1995) Effects of a bleaching agent on human gingival fibroblasts. J Periodontol 66:7–13
Herbert JM, Bono F, Savi P (1996) The mitogenic effect of H2O2 for vascular smooth muscle cells is mediated by an increase of the affinity of basic fibroblast growth factor for its receptor. FEBS Lett 395:43–47
Son YO, Jang YS, Heo JS, Chung WT, Choi KC, Lee JC (2009) Apoptosis-inducing factor plays a critical role in caspase-independent, pyknotic cell death in hydrogen peroxide-exposed cells. Apoptosis 14:796–808
Choe Y, Yu JY, Son YO, Park SM, Kim JG, Shi X, Lee JC (2012) Continuously generated H2O2 stimulates the proliferation and osteoblastic differentiation of human periodontal ligament fibroblasts. J Cell Biochem 113:1426–1436
Yu JY, Lee SY, Son YO, Shi X, Park SS, Lee JC (2012) Continuous presence of H2O2 induces mitochondrial-mediated, MAPK- and caspase-independent growth inhibition and cytotoxicity in human gingival fibroblasts. Toxicol In Vitro 26:561–570
Choi EM, Kim GH, Lee YS (2009) Atractylodes japonica root extract protects osteoblastic MC3T3-E1 cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. Phytother Res 23:1537–1542
Chaves Neto AH, Machado D, Yano CL, Ferreira CV (2011) Antioxidant defense and apoptotic effectors in ascorbic acid and β-glycerophosphate-induced osteoblastic differentiation. Dev Growth Differ 53:88–96
Kim YH, Lee YS, Choi EM (2010) Chrysoeriol isolated from Eurya cilliata leaves protects MC3T3-E1 cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. J Appl Toxicol 30:666–673
Hartmann C (2007) Skeletal development-Wnts are in control. Mol Cells 24:177–184
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209
Liu F, Kohlmeier S, Wang CY (2008) Wnt signaling and skeletal development. Cell Signal 20:999–1009
Gordon MD, Nusse R (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429–22433
Liu J, Wang Y, Du W, Liu W, Liu F, Zhang L, Zhang M, Hou M, Liu K, Zhang S, Yu B (2013) Wnt1 inhibits hydrogen peroxide-induced apoptosis in mouse cardiac stem cells. PLoS One 8:e58883
Shin SY, Kim CG, Jho EH, Rho MS, Kim YS, Kim YH, Lee YH (2004) Hydrogen peroxide negatively modulates Wnt signaling through downregulation of beta-catenin. Cancer Lett 212:225–231
Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, Sylvester KG (2013) Wnt/β-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. J Biol Chem 288:17214–17224
Zhang Q, Pi J, Woods CG, Andersen ME (2010) A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol 244:84–97
Shin SM, Yang JH, Ki SH (2013) Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev 2013:763257
Rana T, Schultz MA, Freeman ML, Biswas S (2012) Loss of Nrf2 accelerates ionizing radiation-induced bone loss by upregulating RANKL. Free Radic Biol Med 53:2298–2307
Hinoi E, Fujimori S, Wang L, Hojo H, Uno K, Yoneda Y (2006) Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J Biol Chem 281:18015–18024
Park CK, Lee Y, Kim KH, Lee ZH, Joo M, Kim HH (2014) Nrf2 is a novel regulator of bone acquisition. Bone 63:36–46
Heo JS, Lee SY, Lee JC (2010) Wnt/β-catenin signaling enhances osteoblastogenic differentiation from human periodontal ligament fibroblasts. Mol Cells 30:449–454
Jeon YM, Kook SH, Rho SJ, Lim SS, Choi KC, Kim HS, Kim JG, Lee JC (2013) Fibroblast growth factor-7 facilitates osteogenic differentiation of embryonic stem cells through the activation of ERK/Runx2 signaling. Mol Cell Biochem 382:37–45
Park SS, Kim KA, Lee SY, Lim SS, Jeon YM, Lee JC (2012) X-ray radiation at low doses stimulates differentiation and mineralization of mouse calvarial osteoblasts. BMB Rep 45:571–576
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ (2004) Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 314:197–207
Liu AL, Zhang ZM, Zhu BF, Liao ZH, Liu Z (2004) Metallothionein protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. Cell Biol Int 28:905–911
Xu ZS, Wang XY, Xiao DM, Hu LF, Lu M, Wu ZY, Bian JS (2011) Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage-implications for the treatment of osteoporosis. Free Radic Biol Med 50:1314–1323
Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945
ten Dijke P, Krause C, de Gorter DJ, Lowik CW, van Bezooijen RL (2008) Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am 90:31–35
Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES (2013) β-catenin is required in odontoblasts for tooth root formation. J Dent Res 92:215–221
Ming M, Wang S, Wu W, Senyuk V, Le Beau MM, Nucifora G, Qian Z (2012) Activation of Wnt/β-catenin protein signaling induces mitochondria-mediated apoptosis in hematopoietic progenitor cells. J Biol Chem 287:22683–22690
Farhana L, Dawson MI, Das JK, Murshed F, Xia Z, Hadden TJ, Hatfield J, Fontana JA (2012) Adamantyl retinoid-related molecules induce apoptosis in pancreatic cancer cells by inhibiting IGF-1R and Wnt/β-catenin pathways. J Oncol 2012:796729
Kostyuk SV, Ermakov AV, Alekseeva AY, Smirnova TD, Glebova KV, Efremova LV, Baranova A, Veiko NN (2012) Role of extracellular DNA oxidative modification in radiation induced bystander effects in human endotheliocytes. Mutat Res 729:52–60
Kim KA, Kook SH, Song JH, Lee JC (2014) A phenolic acid phenethyl urea derivative protects against irradiation-induced osteoblast damage by modulating intracellular redox state. J Cell Biochem 115:1877–1887
Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y (2007) Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 59:27–33
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (KRF) funded by the Ministry of Science, ICT and future Planning (NRF-2013R1A2A2A01967207).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sung-Ho Kook and Daewoo Lee have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kook, SH., Lee, D., Cho, ES. et al. Activation of canonical Wnt/β-catenin signaling inhibits H2O2-induced decreases in proliferation and differentiation of human periodontal ligament fibroblasts. Mol Cell Biochem 411, 83–94 (2016). https://doi.org/10.1007/s11010-015-2570-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2570-4