Abstract
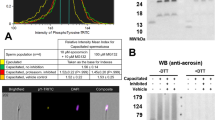
Fertilization, the union of male and female gametes to create offspring, is an intricate biological process dependent upon several biochemical and physiological events. Our understanding of the functions of protein constituents of the outer acrosomal membrane-associated matrix complex (OMC) is limited. A highly purified OMC fraction isolated from bovine cauda sperm heads comprised 54, 50, 45, and 38–19 kDa polypeptides. The objective of this study is to identify and characterize the 45 kDa (OMC45) polypeptide, to define its role in binding acrosomal hydrolases, and to examine the fate of OMC45 polypeptide during the acrosome reaction. We isolated OMC45 polypeptide from the high-pH insoluble fraction of OMC. Proteomic analysis of OMC45 by MALDI-TOF–TOF yielded eight peptides that matched the NCBI database sequence of Tektin 3 (TEKT3). Triton X-100-permeabilized cauda sperm exhibited intense staining of the acrosomal segment with anti-OMC45 and anti-TEKT3. The OMC45 polypeptide was solubilized by radio-immunoprecipitation assay buffer extraction. The solubilized fraction was subjected to immunoprecipitation analysis. The OMC45 polypeptide was recovered in the anti-OMC45 immunoprecipitation pellet. An identical blot stained with anti-TEKT3 exhibited the presence of TEKT3 polypeptide in the anti-OMC45 pellet. Our immunofluorescence and biochemical studies confirm the proteomics identification of OMC45 polypeptide and that it exhibits a sequence similarity to TEKT3. OMC45 glycoprotein possesses both N-linked and O-linked oligosaccharides. Deglycosylated OMC45 revealed a significant reduction in both acrosin and N-acetylglucosaminidase (NAGA) binding in comparison with acrosin and NAGA binding to a native OMC45 polypeptide, demonstrating the important role of oligosaccharides in hydrolase binding. OMC45 polypeptide is not released during the acrosome reaction but remains in the particulate cell subfraction, associated with the hybrid membrane complex.









Similar content being viewed by others
References
Yanagimachi R (1993) Mammalian fertilization. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven Press, New York, pp 189–317
Wassarman PM, Jovine L, Litscher ES (2001) A profile of fertilization in mammals. Nat Cell Biol 3:E59–E64
Primakoff P, Myles DG (2002) Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 296:2183–2185
Kopf GS, Gerton GL (1991) The mammalian sperm acrosome and the acrosome reaction. In: Wassarman PM (ed) Elements of mammalian fertilization. CRC Press, Boca Raton, pp 153–203
Huang TTF, Hardy DM, Yanagimachi H, Teuscher C, Tung K, Wild G, Yanagimachi R (1985) pH and protease control of acrosomal stasis and release during the guinea pig sperm acrosome reaction. Biol Reprod 32:451–462
Talbot P, DiCarlantonio G (1985) Cytochemical localization of dipeptidyl peptidase II (DPP-II) in mature guinea pig sperm. J Histochem Cytochem 33:1169–1172
DiCarlantonio G, Talbot P (1988) Evidence for sequential deployment of secretory enzymes during the normal acrosome reaction of guinea pig sperm in vitro. Gamete Res. 21:425–438
Hyatt H, Gwatkin RBL (1988) Characterization of isolated acrosomal matrices from hamster spermatozoa. J Reprod Fertil 83:419–429
Olson GE, Winfrey VP, Davenport GR (1988) Characterization of matrix domains of the hamster acrosome. Biol Reprod 39:1145–1158
Noland TD, Davis LS, Olson GE (1989) Regulation of proacrosin conversion in isolated guinea pig sperm acrosomal apical segments. J Biol Chem 264:13586–13590
Hardy DM, Oda MN, Friend DS, Huang TTF (1991) A mechanism for differential release of acrosomal enzymes during the acrosome reaction. Biochem J 275:759–766
NagDas SK, Winfrey VP, Olson GE (1996) Identification of hydrolase binding activities of the acrosomal matrix of hamster spermatozoa. Biol Reprod 55:1405–1414
NagDas SK, Winfrey VP, Olson GE (1996) Proacrosin-acrosomal matrix binding interactions in ejaculated bovine spermatozoa. Biol Reprod 54:111–121
Baba T, Niida Y, Michikawa Y, Kasiwabara S, Kodaira K, Takenaka M, Kohno N, Gerton GL, Arai Y (1994) An acrosomal protein, sp32, in mammalian sperm is a binding protein specific for two proacrosins and an acrosin intermediate. J Biol Chem 269:10133–10140
Holt WV (1979) Development and maturation of the mammalian acrosome. A cytochemical study using phosphotungstic acid staining. J Ultrastruct Res 68:58–71
Green DPL (1978) The activation of proteolysis in the acrosome reaction of guinea pig sperm. J Cell Sci 32:153–164
Nuzzo NA, Anderson RA, Zaneveld LJD (1990) Proacrosin activation and acrosin release during the guinea pig acrosome reaction. Mol Reprod Dev 25:52–60
Olson GE, Winfrey VP, Garbers DL, Noland TD (1985) Isolation and characterization of a macromolecular complex associated with the outer acrosomal membrane of bovine spermatozoa. Biol Reprod 33:761–779
Nagdas SK, Hamilton SL, Raychoudhury S (2010) Identification of acrosomal matrix-specific hydrolases binding proteins of bovine cauda epididymal spermatozoa. J Androl 31:177–187
Olson GE, Winfrey VP, Neff JC, Lukas TJ, NagDas SK (1997) An antigenically related polypeptide family is a major structural constituent of a stable acrosomal matrix assembly in bovine spermatozoa. Biol Reprod 57:325–334
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76:4350–4354
Fairbanks G, Steck TL, Wallach DFH (1971) Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2606–2617
Wray W, Boulikas T, Wray VP, Hancock R (1981) Silver staining of proteins in polyacrylamide gels. Anal Biochem 118:197–203
NagDas SK, Winfrey VP, Olson GE (2000) Identification of a hamster epididymal region-specific secretory glycoprotein that binds nonviable spermatozoa. Biol Reprod 63:1428–1436
Parrish JJ, Susko-Parrish J, Winer MA, First NL (1988) Capacitation of bovine sperm by heparin. Biol Reprod 38:1171–1180
Lottenberg R, Christensen U, Jackson CM, Coleman PL (1981) Assay of coagulation proteases using peptide chromogenic and fluorogenic substrates. Methods Enzymol 80:341–361
Miller DJ, Gong X, Shur BD (1993) Sperm require B-N-acetylglucosaminidase to penetrate through the egg zona pellucida. Development 118:1279–1289
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Olson GE, Winfrey VP (1985) Structure of membrane domains and matrix components of the bovine acrosome. J Ultrastruct Res 90:9–25
Takiguchi H, Murayama E, Kaneko T, Kurio H, Toshimori K, Iida H (2011) Characterization and subcellular localization of Tektin 3 in rat spermatozoa. Mol Reprod Dev 78:611–620
Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS (1995) Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121:1129–1137
Porambo JR, Salicioni AM, Visconti PE, Platt MD (2012) Sperm phosphoproteomics: historical perspectives and current methodologies. Expert Rev Proteomics 9:533–548
Saccary L, She YM, Oko R, Kan FW (2013) Hamster oviductin regulates tyrosine phosphorylation of sperm proteins during in vitro capacitation. Biol Reprod 89:1–11
Nagdas SK, Buchanan T, Raychoudhury S (2014) Identification of peroxiredoxin-5 in bovine cauda epididymal sperm. Mol Cell Biochem 387:113–121
Inoue K, Dewar K, Katsanis N, Reiter LT, Lander ES, Devon KL, Wyman DW, Lupski JR, Birren B (2001) The 1.4-Mb CMT1A duplication/HNPP deletion genomic region reveals unique genome architectural features and provides insights into the recent evolution of new genes. Genome Res 11:1018–1033
Chang XJ, Piperno G (1987) Cross-reactivity of antibodies specific for flagellar tektin and intermediate filament subunits. J Cell Biol 104:1563–1568
Steffen W, Linck RW (1989) Relationship between tektins and intermediate filament proteins: an immunological study. Cell Motil Cytoskelet 14:359–371
McLachlan AD, Stewart M (1982) Periodic charge distribution in the intermediate filament proteins desmin and vimentin. J Mol Biol 162:693–698
Parry DA, Strelkov SV, Burkhard P, Aebi U, Herrmann H (2007) Towards a molecular description of intermediate filament structure and assembly. Exp Cell Res 313:2204–2216
Goldie KN, Wedig T, Mitra AK, Aebi U, Herrmann H, Hoenger A (2007) Dissecting the 3-D structure of vimentin intermediate filaments by cryo-electron tomography. J Struct Biol 158:378–385
Iguchi N, Tanaka H, Fujii T, Tamura K, Kaneko Y, Nojima H, Nishimune Y (1999) Molecular cloning of haploid germ cell-specific tektin cDNA and analysis of the protein in mouse testis. FEBS Lett 456:315–321
Larsson M, Norrander J, Gräslund S, Brundell E, Linck R, Ståhl S, Höög C (2000) The spatial and temporal expression of Tekt1, a mouse tektin C homologue, during spermatogenesis suggest that it is involved in the development of the sperm tail basal body and axoneme. Eur J Cell Biol 79:718–725
Xu M, Zhou Z, Cheng C, Zhao W, Tang R, Huang Y, Wang W, Xu J, Zeng L, Xie Y, Mao Y (2001) Cloning and characterization of a novel human TEKTIN1 gene. Int J Biochem Cell Biol 33:1172–1182
Wolkowicz MJ, Naaby-Hansen S, Gamble AR, Reddi PP, Flickinger CJ, Herr JC (2002) Tektin B1 demonstrates flagellar localization in human sperm. Biol Reprod 66:241–250
Roy A, Yan W, Burns KH, Matzuk MM (2004) Tektin 3 encodes an evolutionarily conserved putative testicular microtubules-related protein expressed preferentially in male germ cells. Mol Reprod Dev 67:295–302
Matsuyama T, Honda Y, Doiguchi M, Iida H (2005) Molecular cloning of a new member of TEKTIN family, Tektin 4, located to the flagella of rat spermatozoa. Mol Reprod Dev 72:120–128
Iida H, Honda Y, Matsuyama T, Shibata Y, Inai T (2006) Tektin 4 is located on outer dense fibers, not associated with axonemal tubulins of flagella in rodent spermatozoa. Mol Reprod Dev 73:929–936
Murayama E, Yamamoto E, Kaneko T, Shibata Y, Inai T, Iida H (2008) Tektin 5, a new Tektin family member, is a component of the middle piece of flagella in rat spermatozoa. Mol Reprod Dev 75:650–658
Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM (2009) Tektin 3 is required for progressive sperm motility in mice. Mol Reprod Dev 76:453–459
Oiki S, Hiyama E, Gotoh T, Iida H (2014) Localization of Tektin 1 at both acrosome and flagella of mouse and bull spermatozoa. Zool Sci 31:101–107
Shimasaki S, Yamamoto E, Murayama E, Kurio H, Kaneko T, Shibata Y, Inai T, Iida H (2010) Subcellular localization of Tektin 2 in rat sperm flagellum. Zool Sci 27:755–761
Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, Maekawa M, Nishimune Y (2004) Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol 24:7958–7964
Yamaguchi A, Kaneko T, Inai T, Iida H (2014) Molecular cloning and subcellular localization of Tektin 2-binding protein 1 (Ccdc 172) in rat spermatozoa. J Histochem Cytochem 62:286–297
Mariappa D, Aladakatti RH, Dasari SK, Sreekumar A, Wolkowicz M, Van Der Hoorn F, Seshagiri PB (2010) Inhibition of tyrosine phosphorylation of sperm flagellar proteins, outer dense fiber protein-2 and Tektin-2, is associated with impaired motility during capacitation of hamster spermatozoa. Mol Reprod Dev 77:182–193
Cao W, Ijiri TW, Huang AP, Gerton GL (2011) Characterization of a novel tektin member, TEKT5, in mouse sperm. J Androl 32:55–69
Olson GE, Winfrey VP, NagDas SK (1998) Acrosome biogenesis in the hamster: ultrastructurally distinct matrix regions are assembled from a common precursor polypeptide. Biol Reprod 58:361–370
Reddi PP, Naaby-Hansen S, Aguolnik I, Tsai J-Y, Silver LM, Flickinger CJ, Herr JC (1995) Complementary deoxyribonucleic acid cloning and characterization of mSP-10: the mouse homologue of human acrosomal protein SP-10. Biol Reprod 53:873–881
Liu MS, Aebersold R, Fann CH, Lee CG (1992) Molecular and developmental studies of a sperm acrosome antigen recognized by HS-63 monoclonal antibody. Biol Reprod 46:937–948
Freemerman AJ, Wright RM, Flickinger CJ, Herr JC (1993) Cloning and sequencing of baboon and cynomolgus monkey intraacrosomal protein SP-10: homology with human SP-10 and a mouse sperm antigen (MSA-63). Mol Reprod Dev 34:140–148
Herr JC, Klotz K, Shannon J, Wright RM, Flickinger CJ (1992) Purification and microsequencing of the intra-acrosomal protein SP-10. Evidence that SP-10 heterogeneity results from endoproteolytic processes. Biol Reprod 47:11–20
Westbrook-Case VA, Winfrey VP, Olson GE (1994) A domain-specific 50-kilodalton structural protein of the acrosomal matrix is processed and released during the acrosome reaction in guinea pig. Biol Reprod 51:1–13
Noland TD, Friday BB, Maulit MT, Gerton GL (1994) The sperm acrosomal matrix contains a novel member of the pentaxin family of calcium-dependent binding proteins. J Biol Chem 269:32607–32614
Reid MS, Blobel CP (1994) Apexin, an acrosomal pentaxin. J Biol Chem 269:32615–32620
Barros C, Bedford JM, Franklin LE, Austin CR (1967) Membrane vesiculation as a feature of the mammalian acrosome reaction. J Cell Biol 34:C1–C5
Yanagimachi R, Phillips DM (1984) The status of acrosomal caps immediately before fertilization in vivo. Gamete Res 9:1–19
Foster JA, Klotz KL, Flickinger CJ, Thomas TS, Wright RM, Castillo JR, Herr JC (1994) Human SP-10: acrosomal distribution, processing, and fate after the acrosome reaction. Biol Reprod 51:1222–1231
Acknowledgments
This study was supported by NIH/NIGMS/1SC3GM096875-04, NSF HBCU-UP #1036257, and FSU RISE Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagdas, S.K., Smith, L., Mcnamara, A. et al. Identification and characterization of a bovine sperm acrosomal matrix protein and its mechanism of interaction with acrosomal hydrolases. Mol Cell Biochem 410, 11–23 (2015). https://doi.org/10.1007/s11010-015-2534-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2534-8