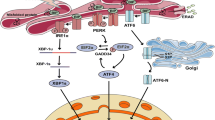
Abstract
Endoplasmic reticulum (ER) stress is emerging as a unifying paradigm and one of the underlying mechanisms in the genesis of diabetes and its complications. While this has prompted the development of ER stress inhibitors, there is a limitation in monitoring of ER stress in vitro and in vivo by reliable methodologies. We validated the secreted alkaline phosphatase (SEAP) activity as a surrogate marker of ER stress in mouse β-TC6 cells exposed to glucolipotoxicity or tunicamycin and studied insulin secretion along with alterations in ER stress markers. SEAP activity assay was measured using the Great EscAPe SEAP kit, insulin levels were determined by Mercodia reagents and mRNA expression of ER stress markers was quantified by real-time PCR. SEAP activity in β-cells was significantly decreased (indicating increased ER stress) on exposure either to glucolipotoxicity or tunicamycin. This was accompanied by an increased mRNA expression of ER stress markers (GRP-78, PERK, IRE1α, ATF6, XBP-1, and CHOP) and decreased insulin secretion. Treating the cells with phenylbutyric acid normalized SEAP activity, decreased mRNA expression of ER stress markers and improved insulin secretion. Interestingly, cells exposed to different classes of anti-diabetes agents or compounds such as resveratrol resisted ER stress. Methylglyoxal also induces ER stress and this was counteracted by aminoguanidine. Out study demonstrates SEAP activity as a novel ER stress monitoring assay to investigate the therapeutic value of agents with ER stress inhibitory potential. Future studies should focus on the exercise of adopting this reporter assay for high-throughput screening mode of drug discovery.






Similar content being viewed by others
Abbreviations
- ATF-6:
-
Activating transcription factor-6
- CHOP:
-
CCAAT/enhancer-binding homologous protein
- GRP-78:
-
Glucose-regulated protein-78
- IRE-1α :
-
Inositol-requiring enzyme-1α
- PERK:
-
PKR-like ER kinase
- XBP-1:
-
X box binding protein1
- PBA:
-
4-Phenylbutyric acid
- SEAP:
-
Secreted alkaline phosphatase
- PDI:
-
Protein disulfide isomerase
- ERO1:
-
ER oxidoreductase1
- INSIG1:
-
Insulin-induced gene1
- SREBP:
-
Sterol regulatory element-binding transcription factor
- AGEs:
-
Advanced Glycation Endproducts
References
Wang S, Kaufman RJ (2012) The impact of the unfolded protein response on human disease. J Cell Biol 197:857–867
Anelli T, Sitia R (2008) Protein quality control in the early secretory pathway. EMBO J 27:315–327
Scheuner D, Kaufman RJ (2008) The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29:317–333
Tersey SA, NishikiY Templin AT et al (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61:818–827
Gujral UP, Narayan KM, Kahn SE, Kanaya AM (2014) The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: the MASALA study. J Diabetes Complicat 28:45–50
Staimez LR, Weber MB, Ranjani H, Ali MK, Echouffo-Tcheugui JB, Phillips LS, Mohan V, Narayan KM (2013) Evidence of reduced β-cell function in Asian Indians with mild dysglycemia. Diabetes Care 36:2772–2778
Thomsen SK, Gloyn AL (2014) The pancreatic β cell: recent insights from human genetics. Trends Endocrinol Metab 25:425–434
Dimas AS, Lagou V, Barker A et al (2014) Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 63:2158–2171
Pasquali L, Gaulton KJ, Rodríguez-Seguí SA et al (2014) Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet 46:136–143
Lee YS, Kim HK, Chung S, Kim KS, Dutta A (2005) Depletion of human micro-RNA miR-125b reveals that it is critical for the proliferation of differentiated cells but not for the down-regulation of putative targets during differentiation. J Biol Chem 280:16635–16641
Hirota M, Kitagaki M, Itagaki H, Aiba S (2006) Quantitative measurement of spliced XBP1 mRNA as an indicator of endoplasmic reticulum stress. J Toxicol Sci 31:149–156
Li F, Hayashi T, Jin G et al (2005) The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain Res 28:59–68
Mao C, Dong D, Little E, Luo S, Lee AS (2004) Transgenic mouse model for monitoring endoplasmic reticulum stress in vivo. Nat Med 10:1013–1014
Berger J, Hauber J, Hauber R, Geiger R, Cullen BR (1988) Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene 66:1–10
Cullen BR, Malim MH (1992) Secreted placental alkaline phosphatase as a eukaryotic reporter gene. Methods Enzymol 216:362–368
Taxis C, Vogel F, Wolf DH (2002) ER-golgi traffic is a prerequisite for efficient ER degradation. Mol Biol Cell 13:1806–1818
Zhang K, Kaufman RJ (2006) The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66:102–109
Minami K, Yano H, Miki T et al (2000) Insulin secretion and differential gene expression in glucose-responsive and unresponsive MIN6 sublines. Am J Physiol Endocrinol Metab 2079:773–781
Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, Mohan V (2011) Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol Cell Biochem 351:197–205
Araki E, Oyadomari S, Mori M (2003) Impact of endoplasmic reticulum stress pathway on pancreatic beta-cells and diabetes mellitus. Exp Biol Med 228:1213–1217
Sparre T, Larsen MR, Heding PE, Karlsen AE, Jensen ON, Pociot F (2005) Unraveling the pathogenesis of type 1 diabetes with proteomics: present and future directions. Mol Cell Proteomics 4:441–457
Sundsten T, Ortsater H (2009) Proteomics in diabetes research. Mol Cell Endocrinol 297:93–103
Dowling P, O’Driscoll L, O’Sullivan F, Dowd A, Henry M, Jeppesen PB, Meleady P, Clynes M (2006) Proteomic screening of glucose-responsive and glucose non-responsive MIN-6 beta cells reveals differential expression of proteins involved in protein folding, secretion and oxidative stress. Proteomics 6:6578–6587
Ahmed M, Bergsten P (2005) Glucose-induced changes of multiple mouse islet proteins analysed by two-dimensional gel electrophoresis and mass spectrometry. Diabetologia 48:477–485
Lu H, Yang Y, Allister EM, Wijesekara N, Wheeler MB (2008) The identification of potential factors associated with the development of type 2 diabetes: a quantitative proteomics approach. Mol Cell Proteomics 7:1434–1451
Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SM, Schuit FC, Jonas JC (2007) Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia 50:1442–1452
Jeffrey KD, Alejandro EU, Luciani DS et al (2008) Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci USA 105:8452–8457
Pirot P, Naamane N, Libert F, Magnusson NE, Orntoft TF, Cardozo AK, Eizirik DL (2007) Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia 50:1006–1014
Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50:752–763
Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A (2006) Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147:3398–3407
Desai K, Wu L (2007) Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Patents Cardiovasc Drug Discov 2:89–99
Vander Jagt DL (2008) Methylglyoxal, diabetes mellitus and diabetic complications. Drug Metab Drug Interact 23:93–124
Wang H, Meng QH, Gordon JR, Khandwala H, Wu L (2007) Proinflammatory and proapoptotic effects of methylglyoxal on neutrophils from patients with type 2 diabetes mellitus. Clin Biochem 40:1232–1239
McLellan AC, Thornalley PJ, Benn J, Sonksen PH (1994) Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 87:21–29
Dhar A, Dhar I, Jiang B, Desai KM, Wu L (2011) Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 60:899–908
Iwawaki T, Akai R, Kohno K, Miura M (2004) A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med 10:98–102
Hiramatsu N, Kasai A, Hayakawa K, Yao J, Kitamura M (2006) Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ESTRAP: evidence for systemic, transient ER stress during endotoxemia. Nucleic Acids Res 34:e93
Ozcan U, Yilmaz E, Özcan L et al (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140
Ozcan U, CaoQ Yilmaz E et al (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461
Kars M, Yang L, Gregor MF et al (2010) Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59:1899–1905
Cadavez L, Montane J, Alcarraz-Vizán G, Visa M, Vidal-Fàbrega Servitja JM, Novials A (2014) Chaperones ameliorate beta cell dysfunction associated with human islet amyloid polypeptide overexpression. PLoS One 9:e101797
Erbay E, Babaev VR, Mayers JR et al (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15:1383–1391
Tveten K, HollaOL Ranheim T, Berge KE, Leren TP, Kulseth MA (2007) 4-Phenylbutyrate restores the functionality of a misfolded mutant low-density lipoprotein receptor. FEBS J 274:1881–1893
Ono K, Ikemoto M, Kawarabayashi T et al (2009) A chemical chaperone, sodium 4-phenylbutyric acid, attenuates the pathogenic potency in human α-synuclein A30P + A53T transgenic mice. Parkinsonism Relat Disord 15:649–654
Ricobaraza A, Cuadrado-Tejedor M, Pérez-Mediavilla A, Frechilla D, Del Río J, García-Osta A (2009) Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 34:1721–1732
Qi W, Mu J, Luo ZF et al (2011) Attenuation of diabetic nephropathy in diabetes rats induced by streptozotocin by regulating the endoplasmic reticulum stress inflammatory response. Metabolism 60:594–603
Zode GS, Kuehn MH, Nishimura DY et al (2011) Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest 121:3542–3553
Xiao C, Giacca A, Lewis GF (2011) Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and β-cell dysfunction in humans. Diabetes 60:918–924
Lily M, Godwin M (2009) Treating prediabetes with metformin systematic review and meta-analysis. Can Fam Phys 55:363–369
Balasubramanyam M (2014) Metformin—Nature’s gift that keeps on giving more. J Obes Metab Res 1:118–120
Simon-Szabó L, Kokas M, Mandl J, Kéri G, Csala M (2014) Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1and apoptosis in rat insulinoma cells. PLoS One 9:97868
Oh YS, Lee YJ, Kang Y, Han J, Lim OK, Jun HS (2013) Exendin-4 inhibits glucolipotoxic ER stress in pancreatic β cells via regulation of SREBP1c and C/EBPβ transcription factors. J Endocrinol 216(3):343–352
Liang CP, Han S, Li G, Tabas I, Tall AR (2012) Impaired MEK signaling and SERCA expression promote ER stress and apoptosis in insulin-resistant macrophages and are reversed by exenatide treatment. Diabetes 61:2609–2620
Shimizu S, Hosooka T, Matsuda T et al (2012) DPP4 inhibitor vildagliptin preserves β-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J Mol Endocrinol 49:125–135
Wu YJ, Guo X, Li CJ, Li DQ, Zhang J, Yang Y, Kong Y, Guo H, Liu DM, Chen LM (2015) Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism. 64:226–235
Szkudelski T, Szkudelska K (2011) Anti-diabetic effects of resveratrol. Ann N Y Acad Sci 1215:34–39
Gertz M, Nguyen GT, Fischer F, Suenkel B, Schlicker C, Franzel B, Tomaschewski J, Aladini F, Becker C, Wolters D, Steegborn C (2012) A molecular mechanism for direct sirtuin activation by resveratrol. PLoS One 7:e49761
Yamabe S, Hirose J, Uehara Y, Okada T, Okamoto N, Oka K, Taniwaki T, Mizuta H (2013) Intracellular accumulation of advanced glycation end products induces apoptosis via endoplasmic reticulum stress in chondrocytes. FEBS J 280:1617–1629
Bronstein I, Fortin J, Stanley PE, Stewart GS, Kricka LJ (1994) Chemiluminescent and bioluminescent reporter gene assays. Anal Biochem 219:169–181
Acknowledgments
This work was supported by research Grants from the Department of Biotechnology (DBT) and the Department of Science and Technology (DST), Government of India, New Delhi, India. RL acknowledges the financial assistance (Senior Research Fellowship) from the Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Balasubramanyam is Dean of Research Studies and Senior Scientist in Madras Diabetes Research Foundation.
Rights and permissions
About this article
Cite this article
Lenin, R., Mohan, V. & Balasubramanyam, M. SEAP activity serves for demonstrating ER stress induction by glucolipotoxicity as well as testing ER stress inhibitory potential of therapeutic agents. Mol Cell Biochem 404, 271–279 (2015). https://doi.org/10.1007/s11010-015-2387-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2387-1