Abstract
The expression level of CC-chemokine receptor 5 (CCR5) is enhanced post inflammatory stimulations and might play a crucial role on inflammatory cells infiltration post myocardial ischemia. The purpose of this study was to evaluate the role of CCR5 on myocardial ischemia–reperfusion (I/R) injury in rats. Adult male rats were randomized to sham group, I/R group (I/R, 30 min coronary artery occlusion followed by 2-h reperfusion), ischemic preconditioning (I/R + Pre), CCR5 antibody group [I/R + CCR5Ab (0.2 mg/kg)], and CCR5 agonist group [I/R + CCR5Ago, RNATES (0.1 mg/kg)], n = 12 each group. The serum level of creatine kinase (CK) and tumor necrosis factor α (TNF-α) were measured by ELISA. Myocardial infarction size and myeloperoxidase (MPO) activity were determined. Myocardial protein expression of CCR5 and intercellular adhesion molecule-1 (ICAM-1) were evaluated by Western blotting and immunohistochemistry staining, respectively. Myocardial nuclear factor-kappa B (NF-κB) activity was assayed by electrophoretic mobility shift assay. Myocardial CCR5 protein expression was significantly reduced in I/R + Pre group (P < 0.05 vs. I/R) and further reduced in I/R + CCR5Ab group (P < 0.05 vs. I/R + Pre). LVSP and ±dP/dt max were significantly lower while serum CK and TNF-α as well as myocardial MPO activity, ICAM-1 expression, and NF-κB activity were significantly higher in I/R group than in sham group (all P < 0.05), which were significantly reversed by I/R + Pre (all P < 0.05 vs. I/R) and I/R + CCR5Ab (all P < 0.05 vs. I/R + Pre) while aggravated by I/R + CCR5Ago (all P < 0.05 vs. I/R). Our results suggest that blocking CCR5 attenuates while enhancing CCR5 aggravates myocardial I/R injury through modulating inflammatory responses in rat heart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reperfusion strategies have substantially contributed to the effective treatment of ischemic heart diseases despite the potential negative impact of ischemia/reperfusion (I/R) injury[1]. I/R injury is characterized by robust local and systemic inflammatory responses which may aggravate tissue injury and adversely affect left ventricular (LV) recovery [2]. A variety of studies in the last decades has shown that the extent of postischemic tissue damage strongly correlates with the number of leukocytes recruited to the reperfused tissue [3–5]. It is known that increased chemokine expression post I/R could promote the adhesion of neutrophils and increase leukocyte infiltration in the cardiac tissue [6], which could serve as one of the important mechanisms mediating the ischemic myocardial damage. Over the past years, chemokines and their receptors have become the subject of intensive investigations and there is a growing body of evidence that chemokines and their receptors are also critically involved in the pathogenesis of I/R. Chemokines are small, secreted proteins that are produced constitutively or in an inducible manner by most cell types and that induce directed cell migration [7]. CC-chemokine receptor 5 (CCR5) is expressed on T-lymphocytes with memory/effector phenotype, macrophages, monocytes, as well as the immature dendritic cells [8]. The expression levels of CCR5 are very low in the mononuclear cells and T cells of human peripheral blood under normal conditions, but could increase significantly after the inflammatory stimulation both in vivo and in vitro [9]. Previous study showed that TAK-779, a small-molecule, nonpeptide compound that selectively binds to a certain subtype of the CC-chemokine receptor, CCR5, with high affinity [10], could effectively reduce leukocyte infiltration of the reperfused tissue and attenuate subsequent postischemic organ failure in mouse models of focal cerebral ischemia [11]. In this study, we tested the hypothesis that myocardial I/R injury could be attenuated by CCR5 antibody or aggravated by CCR5 agonist RANTES in rats.
Materials and methods
Animals and reagents
Healthy adult male Wistar rats (200–250 g) were purchased from Vital River Laboratories, Beijing, China. All experiments were approved by the Institutional Animal Care and Use Committee of Wuhan University. CCR5 antibody and CCR5 agonist RANTES were purchased from Sigma (USA); creatine kinase (CK) and myeloperoxidase (MPO) kit were purchased from Nanjing Jiancheng Bioengineering Institute (China). TNF-α ELISA kit was purchased from Wuhan Boster Bioengineering Co. Ltd. (China). Electrophoretic mobility shift assay (EMSA) kit was purchased from Promega Corp. (Madison, WI, USA).
Surgical preparation
The surgical protocol was performed as described previously [12]. The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (35 mg/kg). After endotracheal intubation with a 14 gauge tube, the rats were then connected to a rodent respirator (TKR-400H, Jiangxi, China, 70 breaths per minute, tidal volume was set to 1.0 ml/100 mg body weight). Body temperature was measured by a rectal thermometer and maintained between 36 and 37 °C by infrared heating lamp. Hemodynamics (left ventricular systolic pressure (LVSP); left ventricular end-diastolic pressure (LVEDP); and heart rate (HR), +dP/dt max and −dP/dt max) were measured through a short segment of saline-filled PE50 tubing which was advanced to left ventricle through right carotid artery and connected to a multi-channel physiological monitoring system (LEAD 2000, Sichuan, China) before left anterior descending coronary artery (LAD) ligation or sham operation. Hemodynamic parameters were obtained immediately after 2-h reperfusion. Electrocardiograph (ECG) leads were connected to the chest and limbs for continuous ECG monitoring throughout the experiment (LEAD 2000, Sichuan, China). Then, the chest was opened via left thoracotomy through the fourth or fifth intercostal space, and the ribs were gently retracted to expose the heart. A 7-0 prolene suture was placed under left anterior descending coronary artery (LAD) after pericardiotomy.
Experimental protocol
Sixty rats were randomly divided into five groups: sham operation group (SHAM, n = 12), I/R group (I/R, n = 12), ischemic preconditioning group (I/R + Pre, n = 12), CCR5 antibody group (I/R + CCR5Ab, n = 12), and CCR5 agonist (RANTES) group (I/R + CCR5Ago, n = 12). Each group was subjected to 30 min of coronary artery occlusion followed by 2 h of reperfusion except sham group. (1) SHAM group: 0.1 ml of anhydrous ethanol was bolus injected through the external jugular vein after thoracotomy and LAD was not ligated; (2) I/R group: LAD ligation for 30 min followed by 2-h reperfusion, 0.1 ml of anhydrous ethanol was bolus injected through the external jugular vein after thoracotomy and after 20-min ischemia; (3) I/R + Pre group: two cycles of 5-min ischemia followed by 5-min reperfusion and one cycle of 10-min ischemia followed by 10-min reperfusion, 0.1 ml of anhydrous ethanol was bolus injected through the external jugular vein before the 3rd circle reperfusion; (4) I/R + CCR5Ab group [13]: LAD ligation for 30 min followed by 2-h reperfusion, 0.2 mg/kg CCR5 antibody diluted in 0.1 ml of anhydrous ethanol was bolus injected through the external jugular vein after thoracotomy and after 20-min ischemia; (5) I/R + CCR5Ag group [14]: LAD ligation for 30 min followed by 2-h reperfusion, 0.1 mg/kg RANTES diluted in 0.1 ml of anhydrous ethanol was bolus injected through the external jugular vein after thoracotomy and after 20-min ischemia. For each individual group, six rats were assigned for myocardial MPO activity determination and measurement of infarct zone and risk area and another 6 rats were assigned for myocardial inflammatory cells counting in HE-stained slices, myocardial CCR5 and ICAM-1 expression, and NF-κB activity determination.
Measurement of infarct zone and risk area
Immediately after hemodynamic measurements, LAD was re-occluded with a 7-0 prolene suture which was used previously at the same place for rats assigned for myocardial MPO activity determination and measurement of infarct zone and risk area, and Evans blue dye (2 ml of a 1 % solution) was injected via the external jugular vein to delineate the area at risk (AAR). The rats were sacrificed under deep pentobarbital anesthesia (60 mg/kg, i.p.) after blood sampling. The heart was then rapidly excised and washed in 0.9 % saline. After removal of the atrium, the ventricle was cut into transverse slices of equal thickness (3 mm) from the apex to the base. The slices were then incubated for 20 min in phosphate-buffered 1 % 2,3,5-triphenyltetrazolium chloride (TTC) at 37 °C, and then fixed in 10 % formalin solution. The AAR was defined as the area not stained with Evans blue dye. The area not stained by TTC was defined as the infarcted zone (AI). The border zones (Evans blue stained area neighboring Evans blue-unstained area), infarcted zones [TTC and The border zones (TTC-stained), infarcted zones (TTC and Evans blue-unstained)], and the nonischemic zones (Evens blue-unstained area remote from Evans blue-unstained area) were photographed and analyzed by the software program Image J 1.36. The AAR, AI, and ventricle size (VS) were assessed by a technician who was blinded to the experimental protocol using computer-assisted planimetry (NIH Image 1.57 software).
The infarct zone, border zones, and risk area were digital recorded. The percentage of the ischemic region (AR) in the whole LV (AR/LV) represents the severity of myocardial ischemia. The percentage of the infarcted region (IS) in the whole ischemic region (AR) (IS/AR) represents the extent of myocardial infarction. The three parts of LV samples (nonischemic zone, border zone, and infract zone) were stored in −80 °C refrigerator for determining MPO activity.
Blood collection of tissue sampling
After hemodynamic measurements, 4-ml blood was obtained from the carotid artery of all rats. Blood samples were placed static for 30 min at room temperature, and then centrifuged at 4,000 r/min for 10 min at 4 °C. The upper serum was removed into new EP tubes and stored in −80 °C refrigerator for detection of CK and TNF-α.
For rats assigned myocardial inflammatory cells counting in HE-stained slices, myocardial CCR5 and ICAM-1 expression, and NF-κB binding activity determination, rats were sacrificed under deep pentobarbital anesthesia (60 mg/kg, i.p.) after blood sampling, hearts were excised and washed with ice-cold saline solution. Two transversal sections (3-mm thick) from the middle part of each heart were prepared and stained with hematoxylin–eosin (HE) for evaluation of the inflammatory response in the cardiac tissues. Severity of inflammatory cell infiltration on HE staining was scored using the following scale [15]: 0 = no inflammation; 1 = cellular infiltrates only around blood vessel and meninges; 2 = mild cellular infiltrates in parenchyma (1–10/section); 3 = moderate cellular infiltrates in parenchyma (11–100/section); 4 = serious cellular infiltrates in parenchyma (100/section).
The remaining LV free wall was divided into three parts. One portion of LV free wall was used for the determination of myocardial ICAM-1 and fixed in 4 % paraformaldehyde. The second portion of LV free wall was stored in −80 °C refrigerator until use for NF-κB binding activity determination. The third portion was stored in −80 °C refrigerator until use for myocardial CCR5 protein expression determination.
Determination of Serum CK, TNF-α, and myocardial MPO activity
Serum CK level was determined by chemical colorimetric method. The serum level TNF-α was determined by rat TNF-α ELISA kit, referring to the manual. Tetramethyl benzidine method was applied for the determination of myocardial MPO activity.
Immunohistochemistry
Immunohistochemical staining of ICAM-1 was performed by the Strept Avidin Biotin Complex (SABC) method. Mouse monoclonal ICAM-1 antibody sc-107 (Santa Cruz Biotechnology, CA) was diluted at 1:100 as primary antibodies. The streptavidin–biotin complex kit was purchased from Wuhan Boster Biological Technology, Ltd. Wuhan, China. All the procedures were carried out according to the manufacturer’s manual. The data of the extent and intensity of staining were obtained using Image Pro Plus Version 6.0 (Media Cybernetics, Bethesda, MD). Five fields of each slice were photographed and analyzed by mean optical density.
Electrophoretic mobility shift assay
EMSA method was used to detect the DNA-binding activities of NF-κB in nuclear extracts. NF-κB oligonucleotide’s sequence was 5′-AGTTGAGGGGACTTTCCCAGGC-3′ and 5′-GCCTGGGAAAGTCCCCTCAACT-3′. Protein-DNA binding assays were performed with 20 μg of nuclear protein. In order to block the unspecific binding, 1 μg of poly (dI-dC) • poly (dI-dC) was added to the samples; then apply the binding medium containing 5 % glycerol, 1 % NP40, 1 mM MgCl2, 50 mM NaCl, 0.5 mM EDTA, 2 mM DTT, and 10 mM Tris/HCl, and with its pH around 7.5. In each reaction, 20,000 cpm of a radiolabeled probe was included. Samples were incubated at room temperature for 20 min. In order to separate the nuclear protein oligonucleotide complex labeled with 32P from free 32P-labeled oligonucleotide, the samples were subjected to electrophoresis through a 5 % native polyacrylamide gel for 2 h in a running buffer containing 50 mM Tris, pH 8.0, 45 mM borate, and 0.5 mM EDTA. After the separation was achieved, the gel was vacuum-dried for autoradiography and exposed to Fuji X-ray film for 24–48 h at −80 °C. The results were analyzed by medical image analysis system, with its gray-scale value representing the activity of NF-κB [16].
Western blotting
Western blotting was conducted to determine protein levels of CCR5 from myocardial tissues. Total cellular membrane proteins were extracted by Plasma Membrane Protein Extraction Kit (Catalog #K268-50) according to Membrane Protein Extraction Protocol. Supernatants were boiled for 10 min in loading buffer, and then separated by SDSPAGE and transferred onto nitrocellulose membranes. After blockage with 5 % skim milk in Tris-buffered saline (TBS) for 1 h at room temperature, the membranes were incubated with primary antibody at 4 °C overnight. After three washings with TBST, (HRP)-labeled secondary antibodies were added and incubated for another 1.5 h on the shaker at room temperature; the blots were then washed two times with TBST. The developed signal was detected using ECL as per the manufacturer’s instructions and exposed to Hyperfilm.
Antibodies against CCR5 were obtained from Cell Signaling Technology (Boston, MA, USA). The antibody against β-actin was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-rabbit and anti-mouse HRP-labeled antibodies and the ECL detection reagents were from Santa Cruz Biotechnology. The X-ray film used for Western-blot analysis was from Kodak (Rochester, NY, USA). Other chemicals and reagents were of analytical grade.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Normality of distribution of all continuous variables was explored by examining skewness, kurtosis, and Q–Q plots. Differences on continuous data among groups were compared using one-way analysis of variance (ANOVA) followed by either Tukey’s or Games-Howell multiple comparison post-hoc tests as appropriate. Variables with non-normal distribution were compared using the non-parametric Mann–Whitney U-statistic test. A P value <0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS, version 20 for Windows.
Results
Mortality
Five rats died post LAD ligation due to malignant arrhythmias and the remaining 55 rats were used in the study and 5–6 rats were examined in each individual group.
Cardiac function
The cardiac function indexes after 2-h reperfusion or sham operation are shown in Table 1. LVSP, +dP/dt max, and −dP/dt max were all significantly lower in I/R group than those in sham group and were significantly higher in I/R + Pre group and I/R + CCR5Ab group while significantly lower in I/R + CCR5Ago group as compared to I/R group. LVEDP was increased in I/R group than those in sham group and were significantly reduced in I/R + Pre group and I/R + CCR5Ab group while significantly higher in I/R + CCR5Ago group as compared to I/R group.
Serum levels of CK and TNF-α
Similarly, serum levels of CK and TNF-α were both significantly higher in I/R group compared to sham group and were significantly reduced in I/R + Pre group and I/R + CCR5Ab groups while significantly increased in I/R + CCR5Ago group compared to I/R group (Fig. 1).
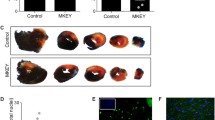
Myocardial infarct size
As shown in Fig. 2, the ischemia region (AR/LV) was similar among group. The myocardial infarct size was significantly smaller in I/R + Pre group and I/R + CCR5Ab group while was significantly larger in I/R + CCR5Ago group than those in I/R group, and was significantly smaller in I/R + CCR5Ab group as compared to I/R + Pre group (P < 0.05).
Myocardial infarct size. The ischemia region (AR/LV) was similar among group. The myocardial infarct size was significantly smaller in I/R + Pre group and I/R + CCR5Ab group while was significantly larger in I/R + CCR5Ago group than those in I/R group, and was significantly smaller in I/R + CCR5Ab group as compared to I/R + Pre group (P < 0.05)
MPO activity in myocardial tissues
MPO activities were significantly increased in I/R group compared to sham group in normal zone; MPO activities were significantly lower in I/R + Pre group and I/R + CCR5Ab group while was significantly higher in I/R + CCR5Ago group in normal, risk and infract zone than in I/R group (Fig. 3).
MPO activity in myocardial tissues. MPO activities were significantly increased in I/R group compared to sham group in normal zone, MPO activities were significantly lower in I/R + Pre group and I/R + CCR5Ab group while was significantly higher in I/R + CCR5Ago group in normal, risk, and infract zone than in I/R group
Inflammatory levels in myocardial tissues
The extent of inflammation of myocardial tissues from LV free wall in different groups was examined in HE-stained transversal myocardial slides. Histopathological images and histological score are listed in Fig. 4. Myocardial tissue in I/R and I/R + CCR5Ago groups presented massive inflammatory cell infiltration as compared to sham group indicating that I/R injury could trigger the inflammatory response which was reduced in I/R + Pre and I/R + CCR5Ab group. Accordingly, histological score was significantly lower in I/R + Pre and I/R + CCR5Ab groups than in I/R group and higher in I/R + CCR5Ago group than in I/R + Pre and I/R + CCR5Ab groups.
Inflammatory levels in myocardial tissues staining by HE (magnification ×400). Representative sections from rats in I/R and I/R + CCR5Ago groups presented massive inflammatory cell infiltration (arrows) as compared to sham group indicating that I/R injury could trigger the inflammatory response which was reduced in I/R + Pre and I/R + CCR5Ab group. Accordingly, histological score was significantly lower in I/R + Pre and I/R + CCR5Ab groups than in I/R group and higher in I/R + CCR5Ago group than in I/R + Pre and I/R + CCR5Ab groups
Myocardial ICAM-1 expression
Brown particles indicated the expression levels of ICAM-1 in myocardial tissues from LV free wall (Fig. 5). Myocardial ICAM-1 expression was significantly upregulated in I/R group compared to sham group and was significantly attenuated in I/R + Pre group and I/R + CCR5Ab group while was significantly increased in I/R + CCR5Ago group compared to that in I/R group.
Myocardial ICAM-1 expression. Brown particles indicated the expression levels of ICAM-1 in myocardial tissues from LV free wall. Myocardial ICAM-1 expression was significantly upregulated in I/R group compared to sham group and was significantly attenuated in I/R + Pre group and I/R + CCR5Ab group while was significantly increased in I/R + CCR5Ago group than in I/R group. (Color figure online)
Myocardial NF-κB binding activity
The myocardial DNA-binding activity of NF-κB was significantly higher in I/R group than in sham group, and was significantly lower in I/R + Pre and I/R + CCR5Ab groups while higher in I/R + CCR5Ago group as compared to I/R group (Fig. 6).
CCR5 protein level of myocardial tissues
We also determined the membrane protein levels of CCR5 in myocardial tissue from LV free wall (Fig. 7). The specific protein expression levels of CCR5 were normalized to β-actin. There is no difference in expression levels of membrane protein CCR5 between sham and I/R groups which was downregulated in I/R + Pre and I/R + CCR5Ab and I/R + CCR5Ago groups.
Discussion
Our study showed that blocking CCR5 attenuates while enhancing CCR5 aggravates myocardial I/R injury through modulating inflammatory responses in rat heart. Thus, strategies modulating CCR5 might serve as potential therapeutic modalities to reducing I/R injury.
Although the precise mechanism of I/R injury has not been fully revealed, a series of studies have demonstrated that I–R could activate the intrinsic inflammatory network. The adhesion and aggregation of neutrophils in the cardiac tissue might be one of the important factors mediating myocardial I/R injury [17]. CCR5 is a receptor for various proinflammatory chemokines. Blocking CCR5 has been proposed as a novel therapeutic approach for cardiovascular conditions by interfering with systemic inflammation. This concept is supported by an animal study by Veillard et al. [18] in which treatment of hypercholesterolemic mice with the CCR5 antagonist Met-RANTES reduced progression of atherosclerosis and CCL5/RANTES inhibition attenuated myocardial reperfusion injury in atherosclerotic mice [14]. Moreover, treatment of apoE-deficient mice with Met-RANTES reduced neointimal plaque area and macrophage infiltration [19] and treatment with TAK-799, a CCR5 chemokine receptor antagonist, reduced lesion development in a collar-induced carotid artery atherosclerosis model [20]. Finally, TAK-779 treatment also reduced leukocyte infiltration and ischemic injury in a mouse model of focal cerebral ischemia [11]. In line with the above findings, we demonstrated that CCR5 antibody effectively reduced myocardial inflammatory cell infiltration and myocardial infarct size in this rat I/R model. It is to note that CCR5 activation was not evidenced in this I/R model; however, our results showed that CCR5 antibody treatment reduced myocardial injury in this model by reducing inflammatory responses. The exact mechanism responsible for the CCR5 antibody treatment effects in this model warrants further studies. Previous studies found that stimulation of increased TNF-α activity could upregulate ICAM-1 expression which then could function as an adhesion molecule promoting neutrophils infiltration [9]. Treatment with specific antibody of ICAM-1 resulted in coronary vascular and myocardial protection as shown by the decrease of myocardial infarct size [21]. Similarly, we showed that treatment with CCR5 antibody significantly reduced the myocardial expression of ICAM-1. In addition, MPO activity (an indicator of neutrophil accumulation in tissue) decreased significantly in both the risk area and infarcted area in I/R + Pre and I/R + CCR5Ab groups while increased in I/R + CCR5Ago group compared with I/R group. Thus, CCR5 antibody reduced while CCR5 agonist enhanced the inflammation in ischemic hearts by down- or upregulating the expression of TNF-α and ICAM-1. Taken together, treatment with CCR5 antibody that reduced infiltration of neutrophils in the ischemic myocardium might contribute to the reduced infarcted size and serum level of CK in this rat I/R model.
Conclusion
In conclusion, our study provides the first evidence that CCR5 antibody could reduce cardiac inflammation and protect the heart from I/R injury via inhibition of the activity of NF-κB, ICAM-1 expression, and MPO activities in this rat I/R model. We propose that targeting CCR5 might serve as a potential novel promising strategy for the treatment of ischemic myocardial disease.
Change history
25 November 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11010-023-04897-8
References
Ribichini F, Wijns W (2002) Acute myocardial infarction: reperfusion treatment. Heart 88(3):298–305
Frangogiannis NG, Smith CW, Entman ML (2002) The inflammatory response in myocardial infarction. Cardiovasc Res 53(1):31–47
Harris AG, Leiderer R, Peer F, Messmer K (1996) Skeletal muscle microvascular and tissue injury after varying durations of ischemia. Am J Physiol 271(6 Pt 2):H2388–H2398
Jolly SR, Kane WJ, Hook BG, Abrams GD, Kunkel SL, Lucchesi BR (1986) Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am Heart J 112(4):682–690
Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR (1983) Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67(5):1016–1023
Frangogiannis NG (2004) The role of the chemokines in myocardial ischemia and reperfusion. Curr Vasc Pharmacol 2(2):163–174
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354(6):610–621. doi:10.1056/NEJMra052723
Oppermann M (2004) Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal 16(11):1201–1210. doi:10.1016/j.cellsig.2004.04.007
Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC (1989) Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest 83(6):2008–2017. doi:10.1172/JCI114111
Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M (1999) A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A 96(10):5698–5703
Reichel CA, Khandoga A, Anders HJ, Schlondorff D, Luckow B, Krombach F (2006) Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol 79(1):114–122. doi:10.1189/jlb.0605337
Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J (2004) Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res 62(1):74–85. doi:10.1016/j.cardiores.2004.01.006
Li J, Xia J, Zhang K, Xu L (2009) Suppression of acute and chronic cardiac allograft rejection in mice by inhibition of chemokine receptor 5 in combination with cyclosporine A. J Surg Res 157(1):81–90. doi:10.1016/j.jss.2009.01.014
Braunersreuther V, Pellieux C, Pelli G, Burger F, Steffens S, Montessuit C, Weber C, Proudfoot A, Mach F, Arnaud C (2010) Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice. J Mol Cell Cardiol 48(4):789–798. doi:10.1016/j.yjmcc.2009.07.029
Okuda Y, Sakoda S, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T (1999) IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35–55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol 101(2):188–196
Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY (2002) Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev 123(12):1589–1595
Saeed SA, Waqar MA, Zubairi AJ, Bhurgri H, Khan A, Gowani SA, Waqar SN, Choudhary MI, Jalil S, Zaidi AH, Ara I (2005) Myocardial ischaemia and reperfusion injury: reactive oxygen species and the role of neutrophil. J Coll Physicians Surg Pak 15(8):507–514
Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F (2004) Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res 94(2):253–261. doi:10.1161/01.RES.0000109793.17591.4E
Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C (2002) Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 106(12):1523–1529
van Wanrooij EJ, Happe H, Hauer AD, de Vos P, Imanishi T, Fujiwara H, van Berkel TJ, Kuiper J (2005) HIV entry inhibitor TAK-779 attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 25(12):2642–2647. doi:10.1161/01.ATV.0000192018.90021.c0
Lefer DJ, Flynn DM, Anderson DC, Buda AJ (1996) Combined inhibition of P-selectin and ICAM-1 reduces myocardial injury following ischemia and reperfusion. Am J Physiol 271(6 Pt 2):H2421–H2429
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited. (http://creativecommons.org/licenses/by/2.0)
About this article
Cite this article
Shen, B., Li, J., Gao, L. et al. RETRACTED ARTICLE: Role of CC-chemokine receptor 5 on myocardial ischemia–reperfusion injury in rats. Mol Cell Biochem 378, 137–144 (2013). https://doi.org/10.1007/s11010-013-1604-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1604-z