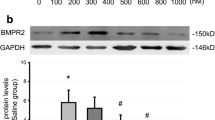
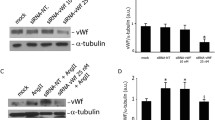
Abstract
Cardiovascular diseases involve critical mechanisms including impaired nitric oxide (NO) levels and abnormal matrix metalloproteinase (MMP) activity. While NO downregulates MMP expression in some cell types, no previous study has examined whether NO downregulates MMP levels in endothelial cells. We hypothesized that NO donors could attenuate MMP-9 production by human umbilical vein endothelial cells (HUVECs) as a result of less NFκB activation or cyclic GMP (cGMP)-mediated mechanisms. We studied the effects of DetaNONOate (10–400 μM) or SNAP (50–400 μM) on phorbol 12-myristate 13-acetate (PMA; 10 nM)-induced increases in MMP-9 activity (by gel zymography) or concentrations (by ELISA) as well as on a tissue inhibitor of MMPs’ (TIMP)-1 concentrations (by ELISA) in the conditioned medium of HUVECs incubated for 24 h with these drugs. We also examined whether the irreversible inhibitor of soluble guanylyl cyclase ODQ modified the effects of SNAP or whether 8-bromo-cGMP (a cell-permeable analog of cGMP) influenced PMA-induced effects on MMP-9 expression. Total and phospho-NFκB p65 concentrations were measured in HUVEC lysates to assess NFκB activation. Both NO donors attenuated PMA-induced increases in MMP-9 activity and concentrations without significantly affecting TIMP-1 concentrations. This effect was not modified by ODQ, and 8-bromo-cGMP did not affect MMP-9 concentrations. While PMA increased phospho-NFκB p65 concentrations, SNAP had no influence on this effect. In conclusion, this study shows that NO donors may attenuate imbalanced MMP expression and activity in endothelial cells independent of cGMP- or NFκB-mediated mechanisms. Our results may offer an important pharmacological strategy to approach cardiovascular diseases.






Similar content being viewed by others
References
Moncada S, Higgs EA (2006) The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147(Suppl 1):S193–S201
Castro MM, Kandasamy AD, Youssef N, Schulz R (2011) Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res 64(6):551–560
Castro MM, Rizzi E, Ceron CS, Guimaraes DA, Rodrigues GJ, Bendhack LM, Gerlach RF, Tanus-Santos JE (2012) Doxycycline ameliorates 2K–1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide 26(3):162–168
Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus-Santos JE, Gerlach RF (2009) Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol 29(3):194–201
Demacq C, Metzger IF, Gerlach RF, Tanus-Santos JE (2008) Inverse relationship between markers of nitric oxide formation and plasma matrix metalloproteinase-9 levels in healthy volunteers. Clin Chim Acta 394(1–2):72–76
Metzger IF, Sandrim VC, Tanus-Santos JE (2012) Endogenous nitric oxide formation correlates negatively with circulating matrix metalloproteinase (MMP)-2 and MMP-9 levels in black subjects. Mol Cell Biochem 360(1–2):393–399
Eberhardt W, Beeg T, Beck KF, Walpen S, Gauer S, Bohles H, Pfeilschifter J (2000) Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int 57(1):59–69
Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J (2000) Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol 165(10):5788–5797
Sinha I, Hannawa KK, Ailawadi G, Woodrum DT, Ford JW, Henke PK, Stanley JC, Eagleton MJ, Upchurch GR Jr (2006) The nitric oxide donor DETA-NONOate decreases matrix metalloproteinase-9 expression and activity in rat aortic smooth muscle and abdominal aortic explants. Ann Vasc Surg 20(1):92–98
Dey NB, Lincoln TM (2012) Possible involvement of Cyclic-GMP-dependent protein kinase on matrix metalloproteinase-2 expression in rat aortic smooth muscle cells. Mol Cell Biochem 368(1–2):27–35
Matthews JR, Botting CH, Panico M, Morris HR, Hay RT (1996) Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res 24(12):2236–2242
Van der Heiden K, Cuhlmann S, le Luong A, Zakkar M, Evans PC (2010) Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 118(10):593–605
Clark IM, Swingler TE, Sampieri CL, Edwards DR (2007) The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 40(6–7):1362–1378
Pfeilschifter J, Eberhardt W, Beck KF (2001) Regulation of gene expression by nitric oxide. Pflugers Arch 442(4):479–486
Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL (1992) Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem 267(7):4583–4591
Izidoro-Toledo TC, Guimaraes DA, Belo VA, Gerlach RF, Tanus-Santos JE (2011) Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells. Naunyn Schmiedebergs Arch Pharmacol 383(6):547–554
Liu HT, Li WM, Xu G, Li XY, Bai XF, Wei P, Yu C, Du YG (2009) Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol Res 59(3):167–175
Gerlach RF, Demacq C, Jung K, Tanus-Santos JE (2007) Rapid separation of serum does not avoid artificially higher matrix metalloproteinase (MMP)-9 levels in serum versus plasma. Clin Biochem 40(1–2):119–123
Gerlach RF, Uzuelli JA, Souza-Tarla CD, Tanus-Santos JE (2005) Effect of anticoagulants on the determination of plasma matrix metalloproteinase (MMP)-2 and MMP-9 activities. Anal Biochem 344(1):147–149
Souza-Tarla CD, Uzuelli JA, Machado AA, Gerlach RF, Tanus-Santos JE (2005) Methodological issues affecting the determination of plasma matrix metalloproteinase (MMP)-2 and MMP-9 activities. Clin Biochem 38(5):410–414
Marcaccini AM, Meschiari CA, Zuardi LR, de Sousa TS, Taba M Jr, Teofilo JM, Jacob-Ferreira AL, Tanus-Santos JE, Novaes AB Jr, Gerlach RF (2010) Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J Clin Periodontol 37(2):180–190
Hah N, Lee ST (2003) An absolute role of the PKC-dependent NF-kappaB activation for induction of MMP-9 in hepatocellular carcinoma cells. Biochem Biophys Res Commun 305(2):428–433
Massaro M, Zampolli A, Scoditti E, Carluccio MA, Storelli C, Distante A, De Caterina R (2010) Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc Res 86(2):311–320
Braam B, de Roos R, Dijk A, Boer P, Post JA, Kemmeren PP, Holstege FC, Bluysen HA, Koomans HA (2004) Nitric oxide donor induces temporal and dose-dependent reduction of gene expression in human endothelial cells. Am J Physiol Heart Circ Physiol 287(5):H1977–H1986
Schankin CJ, Kruse LS, Reinisch VM, Jungmann S, Kristensen JC, Grau S, Ferrari U, Sinicina I, Goldbrunner R, Straube A, Kruuse C (2010) Nitric oxide-induced changes in endothelial expression of phosphodiesterases 2, 3, and 5. Headache 50(3):431–441
Waldow T, Witt W, Weber E, Matschke K (2006) Nitric oxide donor-induced persistent inhibition of cell adhesion protein expression and NFkappaB activation in endothelial cells. Nitric Oxide 15(2):103–113
Munoz G, San Martin R, Farias M, Cea L, Vecchiola A, Casanello P, Sobrevia L (2006) Insulin restores glucose inhibition of adenosine transport by increasing the expression and activity of the equilibrative nucleoside transporter 2 in human umbilical vein endothelium. J Cell Physiol 209(3):826–835
Sparkman L, Boggaram V (2004) Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 287(4):L764–L773
Lee H, Lin CI, Liao JJ, Lee YW, Yang HY, Lee CY, Hsu HY, Wu HL (2004) Lysophospholipids increase ICAM-1 expression in HUVEC through a Gi- and NF-kappaB-dependent mechanism. Am J Physiol Cell Physiol 287(6):C1657–C1666
Galis ZS, Khatri JJ (2002) Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90(3):251–262
Marson BP, Poli de Figueiredo CE, Tanus-Santos JE (2012) Imbalanced matrix metalloproteinases in cardiovascular complications of end-stage kidney disease: a potential pharmacological target. Basic Clin Pharmacol Toxicol 110(5):409–415
Fontana V, Silva PS, Gerlach RF, Tanus-Santos JE (2012) Circulating matrix metalloproteinases and their inhibitors in hypertension. Clin Chim Acta 413(7–8):656–662
Szmitko PE, Wang CH, Weisel RD, Jeffries GA, Anderson TJ, Verma S (2003) Biomarkers of vascular disease linking inflammation to endothelial activation: Part II. Circulation 108(17):2041–2048
Newby AC (2008) Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 28(12):2108–2114
Newby AC (2012) Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol 56(5–6):232–244
Fontana V, Silva PS, Belo VA, Antonio RC, Ceron CS, Biagi C, Gerlach RF, Tanus-Santos JE (2011) Consistent alterations of circulating matrix metalloproteinases levels in untreated hypertensives and in spontaneously hypertensive rats: a relevant pharmacological target. Basic Clin Pharmacol Toxicol 109(2):130–137
Belo VA, Souza-Costa DC, Lana CM, Caputo FL, Marcaccini AM, Gerlach RF, Bastos MG, Tanus-Santos JE (2009) Assessment of matrix metalloproteinase (MMP)-2, MMP-8, MMP-9, and their inhibitors, the tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in obese children and adolescents. Clin Biochem 42(10–11):984–990
Palei AC, Sandrim VC, Cavalli RC, Tanus-Santos JE (2008) Comparative assessment of matrix metalloproteinase (MMP)-2 and MMP-9, and their inhibitors, tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in preeclampsia and gestational hypertension. Clin Biochem 41(10–11):875–880
Phillips PG, Birnby LM, Narendran A, Milonovich WL (2001) Nitric oxide modulates capillary formation at the endothelial cell-tumor cell interface. Am J Physiol Lung Cell Mol Physiol 281(1):L278–L290
Gurjar MV, DeLeon J, Sharma RV, Bhalla RC (2001) Mechanism of inhibition of matrix metalloproteinase-9 induction by NO in vascular smooth muscle cells. J Appl Physiol 91(3):1380–1386
Souza-Costa DC, Zerbini T, Palei AC, Gerlach RF, Tanus-Santos JE (2005) l-arginine attenuates acute pulmonary embolism-induced increases in lung matrix metalloproteinase-2 and matrix metalloproteinase-9. Chest 128(5):3705–3710
Neto-Neves EM, Dias-Junior CA, Uzuelli JA, Pereira RP, Spiller F, Czaikoski PG, Tanus-Santos JE (2011) Sildenafil improves the beneficial hemodynamic effects exerted by atorvastatin during acute pulmonary thromboembolism. Eur J Pharmacol 670(2–3):554–560
Dias-Junior CA, Cau SB, Oliveira AM, Castro MM, Montenegro MF, Gerlach RF, Tanus-Santos JE (2009) Nitrite or sildenafil, but not BAY 41-2272, blunt acute pulmonary embolism-induced increases in circulating matrix metalloproteinase-9 and oxidative stress. Thromb Res 124(3):349–355
Pilz RB, Casteel DE (2003) Regulation of gene expression by cyclic GMP. Circ Res 93(11):1034–1046
He C (1996) Molecular mechanism of transcriptional activation of human gelatinase B by proximal promoter. Cancer Lett 106(2):185–191
Castier Y, Ramkhelawon B, Riou S, Tedgui A, Lehoux S (2009) Role of NF-kappaB in flow-induced vascular remodeling. Antioxid Redox Signal 11(7):1641–1649
Cau S, Guimaraes D, Rizzi E, Ceron C, Souza L, Tirapelli C, Gerlach R, Tanus-Santos J (2011) Pyrrolidine dithiocarbamate downregulates vascular matrix metalloproteinases and ameliorates vascular dysfunction and remodeling in renovascular hypertension. Br J Pharmacol 164(2):372–381
de Winther MP, Kanters E, Kraal G, Hofker MH (2005) Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25(5):904–914
Eberhardt W, el Akool S, Rebhan J, Frank S, Beck KF, Franzen R, Hamada FM, Pfeilschifter J (2002) Inhibition of cytokine-induced matrix metalloproteinase 9 expression by peroxisome proliferator-activated receptor alpha agonists is indirect and due to a NO-mediated reduction of mRNA stability. J Biol Chem 277(36):33518–33528
el Akool S, Kleinert H, Hamada FM, Abdelwahab MH, Forstermann U, Pfeilschifter J, Eberhardt W (2003) Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol Cell Biol 23(14):4901–4916
Acknowledgments
This study was funded by the Fundação de Aparo a Pesquisa do Estado de São Paulo (FAPESP-Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meschiari, C.A., Izidoro-Toledo, T., Gerlach, R.F. et al. Nitric oxide attenuates matrix metalloproteinase-9 production by endothelial cells independent of cGMP- or NFκB-mediated mechanisms. Mol Cell Biochem 378, 127–135 (2013). https://doi.org/10.1007/s11010-013-1602-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1602-1