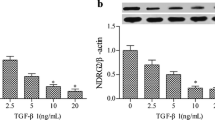
Abstract
Extracellular matrix (ECM) production and epithelial-mesenchymal transition (EMT) are important for phenotypic conversion in normal development and disease states such as tissue fibrosis. Transforming growth factor-β1 (TGFβ1) is one of the most potent inducers of ECM proteins, and its role in the pathogenesis of fibrosis is well established. Ets family is involved in a diverse array of biologic functions including cellular growth, migration, and differentiation. In the present study, we investigated whether Ets-1 has a role in ECM production and EMT in human renal tubuloepithelial cells (HKC cells). TGFβ1 treatment increases Ets-1 expression and nuclear translocation in the HKC cells. Overexpression of recombinant Ets-1 suppressed transcription of α2(I) collagen (COL1A2) and type I collagen production in the TGFβ1-activated HKC cells. From the experiments using specific inhibitors against Smad3 or mitogen-activated protein (MAP) kinase pathways, Ets-1 has an inhibitory role for COL1A2 transcription and the p38 MAPK pathway participates in the negative contribution of Ets-1 in TGFβ1/Smad3-activated renal cells.






Similar content being viewed by others
References
Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC (2003) TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284:F243–F252
Douthwaite JA, Johnson TS, Haylor JL, Watson P, El Nahas AM (1999) Effects of transforming growth factor-beta1 on renal extracellular matrix components and their regulating proteins. J Am Soc Nephrol 10:2109–2119
Hansch GM, Wagner C, Burger A, Dong W, Staehler G, Stoeck M (1995) Matrix protein synthesis by glomerular mesangial cells in culture: effects of transforming growth factor beta (TGF beta) and platelet-derived growth factor (PDGF) on fibronectin and collagen type IV mRNA. J Cell Physiol 163:451–457
Poncelet AC, Schnaper HW (1998) Regulation of human mesangial cell collagen expression by transforming growth factor-beta1. Am J Physiol Renal Physiol 275:F458–F466
Hayashida T, De Caestecker M, Schnaper HW (2003) Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17:1576–1578
Dittmer J, Nordheim A (1998) Ets transcription factors and human diseases. Biochim Biophys Acta 1377:F1–F11
Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P (2005) Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest 115:2508–2516
Naito T, Razzaque MS, Nazneen A, Liu D, Nihei H, Koji T, Taguchi T (2000) Renal expression of the Ets-1 proto-oncogene during progression of rat crescentic glomerulonephritis. J Am Soc Nephrol 11:2243–2255
Reisdorff J, En-Nia A, Stefanidis I, Floege J, Lovett DH, Mertens PR (2002) Transcriptional factor Ets-1 regulates gelatinase A gene expression in mesangial cells. J Am Soc Nephrol 13:1568–1578
Naito T, Tanihata Y, Nishimura H, Tanaka T, Higuchi C, Taguchi T, Sanaka T (2005) Expression of matrix metalloproteinase-9 associated with ets-1 proto-oncogene in rat tubulointestitial cells. Nephrol Dial Transplant 20:2333–2348
Dittmer J (2003) The biology of the Ets1 proto-oncogene. Mol Cancer 3(2):29
Lindemann RK, Nordheim A, Dittmer J (2003) Interfering with TGF-beta-induced Smad3 nuclear accumulation differentially affects TGF-beta-dependent gene expression. Mol Cancer 2:20
Wasylyk B, Wasylyk C, Flores P, Begue A, Leprince D, Stehelin D (1990) The c-Ets proto-oncogene encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature 346:191–193
Lindemann RK, Ballschmieter P, Nordheim A, Dittmer J (2001) Transforming growth factor beta regulates parathyroid hormone-related protein expression in MDA-MB-231 breast cancer cells through a novel Smad/Ets synergism. J Biol Chem 276:46661–46670
Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M (2002) Ets1 is an effector of the transforming growth factor β (TGF-β) signaling pathway and an antagonist of the profibrotic effects of TGF-β. J Biol Chem 277:20399–20408
Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP (1997) Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med 129:318–329
Lamber EP, Vanhille L, Textor LC, Kachalova GS, Sieweke MH, Wilmanns M (2008) Regulation of the transcriptional factor Ets-1 by DNA-mediated homo-dimerization. EMBO J 27:2006–2017
Zawal L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE (1998) Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1:1611–1617
Poncelet AC, De Caestecker MP, Schnaper HW (1999) The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells. Kidney Int 56:1354–1365
Tsuchiyama Y, Wada J, Zhang H, Morita Y, Hiragushi K, Hida K, Shikata K, Yamamura M, Kanwar YS, Makino H (2000) Efficacy of galectins in the ameriolation of nephrotoxic serum nephritis in Wister Kyoto rats. Kidney Int 58:1941–1952
Perone MJ, Bertera S, Shufesky WJ, Divito SJ, Montecalvo A, Mathers AR, Larregina AT, Pang M, Seth N, Wucherpfennig KW, Trucco M, Baum LG, Morelli AE (2009) Suppression of autoimmune diabetes by soluble galectin-1. J Immunol 182:2641–2653
Shimizu M, Khoshnoodi J, Akimoto Y, Kawakami H, Hirano H, Higashihara E, Hosoyamada M, Sekine Y, Kurayama H, Joh K, Hirabayashi J, Kasai K, Tryggvason K, Ito N, Yan K (2009) Expression of galectin-1, a new component of slit diaphragm, is altered in minimal change nephritic syndrome. Lab Invest 89:178–195
Xu J, Lamouille S, Derynck R (2009) TGF-β-induced epithelial to mesenchymal transition. Cell Res 19:156–172
Liu Y (2010) New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21:212–222
Buttice G, Duterque-Coquillaud M, Basuyaux JP, Carrere S, Kurkinen M, Stehelin D (1996) Erg, an Ets-family member, differentially regulates human collagenase 1 (MMP1) and stromelysin1 (MMP3) gene expression by physically interacting with the Fos/Jun complex. Oncogene 13:2297–2306
Pearse DD, Tian RX, Nigro J, Iorgulescu JB, Puzis L, Jaimes EA (2008) Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells. Am J Physiol Renal Physiol 294:F1094–F1100
Tanaka K, Oda N, Iwasaka C, Abe M, Sato Y (1998) Induction of Ets-1 in endothelial cells during reendothelialization after denuding injury. J Cell Physiol 176:235–244
Ghosh AK, Yuan W, Mori Y, Varga J (2000) Smad-dependent stimulation of type 1 collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 19:3546–3555
Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F (2000) Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation on alpha 2(I)-collagen (COL1A2) transcription. J Biol Chem 275:39237–39245
Poncelet AC, Schnaper HW (2001) Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. J Biol Chem 276:6983–6992
Shirakihara T, Saitoh M, Miyazono K (2007) Differential regulation of epithelial and mesenchymal markers by dEF1 proteins in epithelial mesenchymal transition induced by TGF-β. Mol Bio Cell 18:3533–3544
Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, Hume DA, Maki RA, Ostrowski MC (1996) Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol 16:538–547
Koinuma D, Tsutsumi S, Kamimura N, Taniguchi H, Miyazawa K, Sunamura M, Imamura T, Miyazono K, Aburatani H (2009) Chromatin immunoprecipitation on microarray analysis of Smad2/3 binding sites reveals roles of ETS1 and TFAP2A in transforming growth factor β signaling. Mol Cell Biol 29:172–186
Seidel JJ, Graves BJ (2002) An ERK2 docking site in the pointed domain distinguishes a subset of Ets transcription factors. Gene Dev 16:127–137
Yang J, Liu Y (2001) Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal intestinal fibrosis. Am J Pathol 159:1465
Taki M, Verschueren K, Yokoyama K, Nagayama M, Kamata N (2006) Involvement of Ets-1 transcription factor in inducing matrix metalloproteinase-2 expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Int J Oncol 28:487–496
Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beuq H, Mikulits W (2002) Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-β1 and Ha-Ras: steps towards invasiveness. J Cell Sci 115:1189–1202
Disclosure
This study was not sponsored by any groups. We have no commercial conflict in connection with this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
ETS-1 increases TGF-β1-mediated renal fibrosis.
Rights and permissions
About this article
Cite this article
Okano, K., Hibi, A., Miyaoka, T. et al. Inhibitory effects of the transcription factor Ets-1 on the expression of type I collagen in TGF-β1-stimulated renal epithelial cells. Mol Cell Biochem 369, 247–254 (2012). https://doi.org/10.1007/s11010-012-1388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1388-6