Abstract
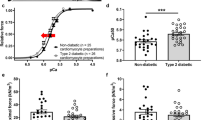
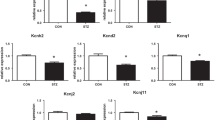
There has been a spectacular rise in the global prevalence of type 2 diabetes mellitus and cardiovascular complications are the major cause of morbidity and mortality in diabetic patients. The objective of the study was to investigate ventricular myocyte shortening, intracellular Ca2+ signalling and expression of genes encoding cardiac muscle proteins in the aged Zucker diabetic fatty (ZDF) rat. There was a fourfold elevation in non-fasting blood glucose in ZDF rats (478.43 ± 29.22 mg/dl) compared to controls (108.22 ± 2.52 mg/dl). Amplitude of shortening, time to peak (TPK) and time to half (THALF) relaxation of shortening were unaltered in ZDF myocytes compared to age-matched controls. Amplitude and THALF decay of the Ca2+ transient were unaltered; however, TPK Ca2+ transient was prolonged in ZDF myocytes (70.0 ± 3.2 ms) compared to controls (58.4 ± 2.3 ms). Amplitude of the L-type Ca2+ current was reduced across a wide range of test potentials (−30 to +40 mV) in ZDF myocytes compared to controls. Sarcoplasmic reticulum Ca2+ content was unaltered in ZDF myocytes compared to controls. Expression of genes encoding cardiac muscle proteins, membrane Ca2+ channels, and cell membrane ion transport and intracellular Ca2+ transport proteins were variously altered. Myh6, Tnnt2, Cacna2d3, Slc9a1, and Atp2a2 were downregulated while Myl2, Cacna1g, Cacna1h, and Atp2a1 were upregulated in ZDF ventricle compared to controls. The results of this study have demonstrated that preserved ventricular myocyte shortening is associated with altered mechanisms of Ca2+ transport and a changing pattern of genes encoding a variety of Ca2+ signalling and cardiac muscle proteins in aged ZDF rat.








Similar content being viewed by others
References
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–1053
Zimmet PZ, Alberti KG (2006) Introduction: globalization and the non-communicable disease epidemic. Obesity (Silver Spring) 14(1):1–3
Julien J (1997) Cardiac complications in non-insulin-dependent diabetes mellitus. J Diabetes Complicat 11:123–130
von Bibra H, Thrainsdottir IS, Hansen A, Dounis V, Malmberg K, Ryden L (2005) Tissue Doppler imaging for the detection and quantitation of myocardial dysfunction in patients with type 2 diabetes mellitus. Diabetes Vasc Dis Res 2(1):24–30
Sharman JE, Haluska BA, Fang ZY, Prins JB, Marwick TH (2007) Association of arterial wave properties and diastolic dysfunction in patients with type 2 diabetes mellitus. Am J Cardiol 99(6):844–848
Dounis V, Siegmund T, Hansen A, Jensen J, Schumm-Draeger PM, von Bibra H (2006) Global myocardial perfusion and diastolic function are impaired to a similar extent in patients with type 2 diabetes mellitus and in patients with coronary artery disease—evaluation by contrast echocardiography and pulsed tissue Doppler. Diabetologia 49(11):2729–2740
Markuszewski L, Grycewicz T, Pietruszynski R, Michalkiewicz D, Roszczyk N (2006) Glycosylated hemoglobin and left ventricular diastolic dysfunction in patients with type 2 diabetes mellitus. Pol Merkur Lek 21(121):8–11
Clark JB, Palmer CJ, Shaw WN (1983) The diabetic Zucker fatty rat. Proc Soc Exp Biol Med 173(1):68–75
Shimabukuro M, Zhou YT, Levi M, Unger RH (1998) Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95(5):2498–2502
Zawalich WS, Zawalich KC, Kelley GG, Shulman GI (1995) Islet phosphoinositide hydrolysis and insulin secretory responses from prediabetic fa/fa ZDF rats. Biochem Biophys Res Commun 209(3):974–980
Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, Tang J, Sun X, Polonsky KS, Bell GI (1995) Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes 44(12):1447–1457
Howarth FC, Qureshi MA, Hassan Z, Al Kury LT, Isaev D, Parekh K, Yammahi SR, Oz M, Adrian TE, Adeghate E (2011) Changing pattern of gene expression is associated with ventricular myocyte dysfunction and altered mechanisms of Ca2+ signalling in young type 2 Zucker diabetic fatty rat heart. Exp Physiol 96(3):325–337
Howarth FC, Qureshi MA, White E (2002) Effects of hyperosmotic shrinking on ventricular myocyte shortening and intracellular Ca(2+) in streptozotocin-induced diabetic rats. Pflüg Arch 444(3):446–451
Howarth FC, Calaghan SC, Boyett MR, White E (1999) Effect of the microtubule polymerizing agent taxol on contraction, Ca2+ transient and L-type Ca2+ current in rat ventricular myocytes. J Physiol 516(Pt 2):409–419
Bassani JW, Yuan WL, Bers DM (1995) Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol 37:C1313–C1319
Levi AJ, Hancox JC, Howarth FC, Croker J, Vinnicombe J (1996) A method for making rapid changes of superfusate whilst maintaining temperature at 37 degrees C. Pflüg Arch 432(5):930–937
Spurgeon HA, DuBell WH, Stern MD, Sollott SJ, Ziman BD, Silverman HS, Capogrossi MC, Talo A, Lakatta EG (1992) Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol 447:83–102
Howarth FC, Qureshi MA (2008) Myofilament sensitivity to Ca2+ in ventricular myocytes from the Goto-Kakizaki diabetic rat. Mol Cell Biochem 315(1–2):69–74
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76(9):4350–4354
Raza H, John A (2004) Glutathione metabolism and oxidative stress in neonatal rat tissues from streptozotocin-induced diabetic mothers. Diabetes Metab Res Rev 20(1):72–78
Ross FM, Gwyn P, Spanswick D, Davies SN (2000) Carbenoxolone depresses spontaneous epileptiform activity in the CA1 region of rat hippocampal slices. Neuroscience 100(4):789–796
Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, Burstow D, Brown L (2003) The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ 12(1):44–50
Howarth FC, Jacobson M, Shafiullah M, Adeghate E (2005) Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol 90(6):827–835
Marsh SA, Powell PC, Agarwal A, Dell’Italia LJ, Chatham JC (2007) Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: role of hydronephrosis. Am J Physiol 293(1):H292–H298
van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, Ouwens DM, Diamant M (2009) Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc Diabetol 8:39
Fredersdorf S, Thumann C, Ulucan C, Griese DP, Luchner A, Riegger GA, Kromer EP, Weil J (2004) Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc Pathol 13(1):11–19
Wang P, Chatham JC (2004) Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am J Physiol Endocrinol Metab 286(5):E725–E736
Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC (2007) Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol 292(4):C1370–C1378
Hovnanian A (2007) SERCA pumps and human diseases. Subcell Biochem 45:337–363
Talukder MA, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL (2007) Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. Am J Physiol 293(4):H2418–H2428
Zhao W, Frank KF, Chu G, Gerst MJ, Schmidt AG, Ji Y, Periasamy M, Kranias EG (2003) Combined phospholamban ablation and SERCA1a overexpression result in a new hyperdynamic cardiac state. Cardiovasc Res 57(1):71–81
Ramirez CD, Padron R (2004) Familial hypertrophic cardiomyopathy: genes, mutations and animal models. a review. Invest Clin 45(1):69–99
Fokstuen S, Lyle R, Munoz A, Gehrig C, Lerch R, Perrot A, Osterziel KJ, Geier C, Beghetti M, Mach F, Sztajzel J, Sigwart U, Antonarakis SE, Blouin JL (2008) A DNA resequencing array for pathogenic mutation detection in hypertrophic cardiomyopathy. Hum Mutat 29(6):879–885
Becker JR, Deo RC, Werdich AA, Panakova D, Coy S, Macrae CA (2011) Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish. Dis Model Mech 4(3):400–410
Morales A, Pinto JR, Siegfried JD, Li D, Norton N, Hofmeyer M, Vallin M, Morales AR, Potter JD, Hershberger RE (2010) Late onset sporadic dilated cardiomyopathy caused by a cardiac troponin T mutation. Clin Transl Sci 3(5):219–226
Malo ME, Fliegel L (2006) Physiological role and regulation of the Na+/H+ exchanger. Can J Physiol Pharmacol 84(11):1081–1095
Flack KD, Davy KP, Hulver MW, Winett RA, Frisard MI, Davy BM (2010) Aging, resistance training, and diabetes prevention. J Aging Res 2011:127315
Howlett SE (2010) Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am J Physiol 298(2):H659–H670
Zhang L, Zhang WH, Zhang L, Wang PY (2011) Prevalence of overweight/obesity and its associations with hypertension, diabetes, dyslipidemia, and metabolic syndrome: a survey in the suburban area of Beijing, 2007. Obes Facts 4(4):284–289
Minhas KM, Khan SA, Raju SV, Phan AC, Gonzalez DR, Skaf MW, Lee K, Tejani AD, Saliaris AP, Barouch LA, O’Donnell CP, Emala CW, Berkowitz DE, Hare JM (2005) Leptin repletion restores depressed {beta}-adrenergic contractility in ob/ob mice independently of cardiac hypertrophy. J Physiol 565(Pt 2):463–474
Acknowledgments
This study was supported by a grant from Sheikh Hamdan Bin Rashid Al Maktoum Award for Medical Sciences and from Faculty of Medicine & Health Sciences, UAE University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Howarth, F.C., Qureshi, M.A., Hassan, Z. et al. Contractility of ventricular myocytes is well preserved despite altered mechanisms of Ca2+ transport and a changing pattern of mRNA in aged type 2 Zucker diabetic fatty rat heart. Mol Cell Biochem 361, 267–280 (2012). https://doi.org/10.1007/s11010-011-1112-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1112-y