Abstract
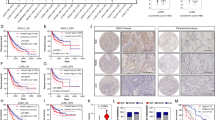
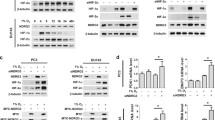
The prolyl hydroxylase domain enzymes (PHDs) play the most notable role in cellular oxygen sensing and oxygen homeostasis, the transcription of PHD genes are involved in the protection against hypoxia and oxidative stress. Intratumoral hypoxia exists in malignant solid tumors primarily due to rapid cancer cell proliferation with high metabolic demands and defective structural and functional vasculature. Previous studies have demonstrated that all the three PHDs have the ability to hydroxylate hypoxia inducible factor (HIF) polypeptides, which are the key molecules in maintaining the oxygen homeostasis. However, PHDs play multiple physiological and pathological roles. There is scant data regarding expression of PHDs genes in non-small cell lung cancer (NSCLC) tissues. In Addition, the relationship between PHDs and apoptosis has never been explored in NSCLC. In this article, we examined the expression of PHD genes and their relationship with the tumor behavior and apoptosis-associated factors in NSCLC. Our results indicated that the expression of PHDs was much higher in lung cancer tissue than that of adjacent normal tissue, and the high expression of PHD3 was associated with early tumor stage and well differentiation in NSCLC. Moreover, increased PHD3 expression was significantly correlated with the low expression of Bcl-2, suggesting its potential role in inducing apoptosis.



Similar content being viewed by others
References
Vaupel P (2004) Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 14:198–206
Vaupel P, Mayer A (2007) Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 26:225–239
Yasuda H (2008) Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: nitric oxide donor as a therapeutic enhancer. Nitric Oxide 19:205–216
Hofer T, Wenger H, Gassmann M (2002) Oxygen sensing, HIF-1alpha stabilization and potential therapeutic strategies. Pflugers Arch 443:503–507
Shinojima T, Oya M, Takayanagi A, Mizuno R, Shimizu N, Murai M (2007) Renal cancer cells lacking hypoxia inducible factor (HIF)-1alpha expression maintain vascular endothelial growth factor expression through HIF-2alpha. Carcinogenesis 28:529–536
Swietach P, Vaughan-Jones RD, Harris AL (2007) Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev 26:299–310
Calvani M, Trisciuoglio D, Bergamaschi C, Shoemaker RH, Melillo G (2008) Differential involvement of vascular endothelial growth factor in the survival of hypoxic colon cancer cells. Cancer Res 68:285–291
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337–1340
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54
Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH (2008) Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood 111:3229–3235
Rantanen K, Pursiheimo J, Hogel H, Himanen V, Metzen E, Jaakkola PM (2008) Prolyl hydroxylase PHD3 activates oxygen-dependent protein aggregation. Mol Biol Cell 19:2231–2240
Reed JC (1997) Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol 34:9–19
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Silvestrini R, Veneroni S, Daidone MG, Benini E, Boracchi P, Mezzetti M, Di Fronzo G, Rilke F, Veronesi U (1994) The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst 86:499–504
Giatromanolaki A, Koukourakis MI, Pezzella F, Turley H, Sivridis E, Bouros D, Bougioukas G, Harris AL, Gatter KC (2008) Expression of prolyl-hydroxylases PHD-1, 2 and 3 and of the asparagine hydroxylase FIH in non-small cell lung cancer relates to an activated HIF pathway. Cancer Lett 262:87–93
Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D (2005) Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest 127:266–274
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279:38458–38465
Berra E, Ginouves A, Pouyssegur J (2006) The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signalling. EMBO Rep 7:41–45
Ginouves A, Ilc K, Macias N, Pouyssegur J, Berra E (2008) PHDs overactivation during chronic hypoxia “desensitizes” HIFalpha and protects cells from necrosis. Proc Natl Acad Sci USA 105:4745–4750
Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH (2006) Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem 281:23482–23491
Gossage L, Zaitoun A, Fareed KR, Turley H, Aloysius M, Lobo DN, Harris AL, Madhusudan S (2010) Expression of key hypoxia sensing prolyl-hydroxylases PHD1, -2 and -3 in pancreaticobiliary cancer. Histopathology 56:908–920
Fu J, Menzies K, Freeman RS, Taubman MB (2007) EGLN3 prolyl hydroxylase regulates skeletal muscle differentiation and myogenin protein stability. J Biol Chem 282:12410–12418
Irwin R, LaPres JJ, Kinser S, McCabe LR (2007) Prolyl-hydroxylase inhibition and HIF activation in osteoblasts promotes an adipocytic phenotype. J Cell Biochem 100:762–772
Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J (2010) Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKbeta independent of hydroxylase activity. Gastroenterology 138:606–615
Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT, Giaccia AJ (2009) Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell 15:527–538
Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, Farese RV, Freeman RS, Carter BD, Kaelin WG Jr, Schlisio S (2005) Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell 8:155–167
Tennant DA, Gottlieb E (2010) HIF prolyl hydroxylase-3 mediates alpha-ketoglutarate-induced apoptosis and tumor suppression. J Mol Med 88:839–849
Liu Y, Huo Z, Yan B, Lin X, Zhou ZN, Liang X, Zhu W, Liang D, Li L, Zhao H, Sun Y, Chen YH (2010) Prolyl hydroxylase 3 interacts with Bcl-2 to regulate doxorubicin-induced apoptosis in H9c2 cells. Biochem Biophys Res Commun 401:231–237
Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA 3rd (2006) Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ Res 98:133–140
Terkhorn SP, Bohensky J, Shapiro IM, Koyama E, Srinivas V (2007) Expression of HIF prolyl hydroxylase isozymes in growth plate chondrocytes: relationship between maturation and apoptotic sensitivity. J Cell Physiol 210:257–265
Acknowledgments
This work was supported, in part, by the National Natural Science Foundation of China (30800404, 30870524), Shanghai leading academic discipline project (B115), Shanghai municipal natural science fund (06DZ19503), and Basic-clinical medicine grant (to H-Q C), Shanghai Rising-Star Program (09QA1401200), Young investigator grant from the Department of Education in China, the Science and Technology Commission of Shanghai Municipality, IBS Bio-medicine grant from Fudan University (to J. Z.). The authors thank Shannon L. Wyszomierski PhD for the critical review and writing assistance of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sufeng Chen, Jie Zhang and Xuebing Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, S., Zhang, J., Li, X. et al. The expression of prolyl hydroxylase domain enzymes are up-regulated and negatively correlated with Bcl-2 in non-small cell lung cancer. Mol Cell Biochem 358, 257–263 (2011). https://doi.org/10.1007/s11010-011-0976-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0976-1