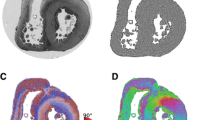
Abstract
Reports describing production of reactive oxygen species in neonatal heart are missing. As lysyl oxidase is potentially important source of H2O2, we studied its role during ontogenic development of rat heart. H2O2 was detected in thin sections of developing rat heart by fluorescence microscopy with the use of fluorescence probe 2′-7′-dichlorofluorescin. The experimental design comprised foetuses 21 days after conception, and then the animals sampled on the 1st, 4th, 7th, 10th, 15th, 30th and 60th day after birth. We also used 7-month-old animals as an example of ageing effects. Since the day 4 on, H2O2 was produced only extracellularly up to the day 15, between days 30 and 60 intracellular production was detected as well, and in 7-month-old animals only extracellular production was observed. The specific inhibitors of lysyl oxidase almost completely quenched the H2O2-dependent fluorescence. Starting from day 7, blue autofluorescence specific to oxidized proteins developed in the vessel wall. Intracellular blue autofluorescence specific to autoxidation products developed after day 30. Chloroform extraction diminished the intracellular blue fluorescence, leaving the extracellular fluorescence intact. This confirmed the protein nature of the fluorophores. Lysyl oxidase is significant source of H2O2 in the heart vessel wall during development and H2O2 oxidatively modifies elastin producing protein blue autofluorescence.





Similar content being viewed by others
References
Li F, Wang X, Capasso JM et al (1996) Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 28:1737–1746
Heron MI, Kuo C, Rakusan K (1999) Arteriolar growth in the postnatal rat heart. Microvasc Res 58:183–186
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Hudlicka O, Wright AJ, Ziada AM (1986) Angiogenesis in the heart and skeletal muscle. Can J Cardiol 2:120–123
Tomanek RJ (2005) Formation of coronary vasculature during development. Angiogenesis 8:273–284
Karnik SK, Brooke BS, Antonio BG et al (2003) A critical role for elastin signaling in vascular morphogenesis and disease. Development 130:411–423
Rodriguez C, Martínez-Gonzáles J, Raposo B et al (2008) Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res 79:7–13
Swee MH, Parks WC, Pierce RA (1995) Developmental regulation of elastin production. J Biol Chem 270:14899–14906
Skoumalová A, Herget J, Wilhelm J (2008) Hypercapnia protects erythrocytes against free radical damage by hypoxia in exposed rats. Cell Biochem Funct 26:801–807
Fukuzawa K, Kishikawa K, Tokumura A et al (1985) Fluorescent pigments by covalent binding of lipid peroxidation by-products to protein and amino acids. Lipids 20:854–861
Goto S, Nakamura A (1997) Age-associated, oxidatively modified proteins: a critical evaluation. Age 20:81–89
Ostadalova I, Vobecky M, Chvojková Z et al (2007) Selenium protects the immature rat heart against ischemia/reperfusion injury. Mol Cell Biochem 300:259–267
Wilhelm J, Vytasek R, Ostadalova I et al (2009) Evaluation of different methods detecting intracellular generation of free radicals. Mol Cell Biochem 328:167–176
Gacheru SN, Trackman PC, Shah MA et al (1990) Structural and catalytic properties of copper lysyl oxidase. J Biol Chem 265:19022–19027
Tang S-S, Trackman PC, Kagan HM (1983) Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem 259:4331–4338
Keston AS, Brandt R (1965) The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem 11:1–5
Babicky A, Ostadalova I, Parizek J et al (1970) Use of radioisotope techniques for determining the weaning period in experimental animals. Physiol Bohemoslovaca 19:457
Bačáková L, Wilhelm J, Herget J et al (1997) Oxidized collagen I stimulates proliferation of vascular smooth muscle cells. Exp Mol Pathol 64:185–194
González JM, Briones AM, Starcher B et al (2005) Influence of elastin on rat small artery mechanical properties. Exp Physiol 90:463–468
Acknowledgments
The study was supported by Grant Agency of Czech Republic, grant no. P303/11/0298.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilhelm, J., Ošt’ádalová, I., Vytášek, R. et al. Generation of hydrogen peroxide in the developing rat heart: the role of elastin metabolism. Mol Cell Biochem 358, 215–220 (2011). https://doi.org/10.1007/s11010-011-0937-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0937-8