Abstract
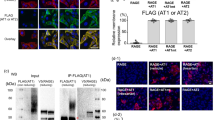
Guanylyl cyclases (GCs), a ubiquitous family of enzymes that metabolize GTP to cyclic GMP (cGMP), are traditionally divided into membrane-bound forms (GC-A-G) that are activated by peptides and cytosolic forms that are activated by nitric oxide (NO) and carbon monoxide. However, recent data has shown that NO activated GC’s (NOGC) also may be associated with membranes. In the present study, interactions of guanylyl cyclase A (GC-A), a caveolae-associated, membrane-bound, homodimer activated by atrial natriuretic peptide (ANP), with NOGC, a heme-containing heterodimer (α/β) β1 isoform of the β subunit of NOGC (NOGCβ1) was specifically focused. NOGCβ1 co-localized with GC-A and caveolin on the membrane in human kidney (HK-2) cells. Interaction of GC-A with NOGCβ1 was found using immunoprecipitations. In a second set of experiments, the possibility that NOGCβ1 regulates signaling by GC-A in HK-2 cells was explored. ANP-stimulated membrane guanylyl cyclase activity (0.05 ± 0.006 pmol/mg protein/5 min; P < 0.01) and intra cellular GMP (18.1 ± 3.4 vs. 1.2 ± 0.5 pmol/mg protein; P < 0.01) were reduced in cells in which NOGCβ1 abundance was reduced using specific siRNA to NOGCβ1. On the other hand, ANP-stimulated cGMP formation was increased in cells transiently transfected with NOGCβ1 (530.2 ± 141.4 vs. 26.1 ± 13.6 pmol/mg protein; P < 0.01). siRNA to NOGCβ1 attenuated inhibition of basolateral Na/K ATPase activity by ANP (192 ± 22 vs. 92 ± 9 nmol phosphate/mg protein/min; P < 0.05). In summary, the results show that NOGCβ1 and GC-A interact and that NOGCβ1 regulates ANP signaling in HK-2 cells. The results raise the novel possibility of cross-talk between NOGC and GC-A signaling pathways in membrane caveolae.




Similar content being viewed by others
References
Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52:375–414
Foster DC, Wedel BJ, Robinson SW, Garbers DL (1999) Mechanisms of regulation and functions of guanylyl cyclases. Rev Physiol Biochem Pharmacol 135:1–39
Kuhn M (2003) Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res 93:700–709
Ruskoaho H (1992) Atrial natriuretic peptide: synthesis, release, and metabolism. Pharmacol Rev 44:479–602
Wilson EM, Chinkers M (1995) Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry 34:4696–4701
Duda T, Goraczniak RM, Sharma RK (1994) Glutamic acid-332 residue of the type C natriuretic peptide receptor guanylate cyclase is important for signaling. Biochemistry 33:7430–7433
Thompson DK, Garbers DL (1995) Dominant negative mutations of the guanylyl cyclase-A receptor. Extracellular domain deletion and catalytic domain point mutations. J Biol Chem 270:425–430
Chinkers M, Wilson EM (1992) Ligand-independent oligomerization of natriuretic peptide receptors. Identification of heteromeric receptors and a dominant negative mutant. J Biol Chem 267:18589–18597
Lowe DG (1992) Human natriuretic peptide receptor-A guanylyl cyclase is self-associated prior to hormone binding. Biochemistry 31:10421–10425
De Lean A, McNicoll N, Labrecque J (2003) Natriuretic peptide receptor A activation stabilizes a membrane-distal dimer interface. J Biol Chem 278:11159–11166
Doyle DD, Ambler SK, Upshaw-Earley J, Bastawrous A, Goings GE, Page E (1997) Type B atrial natriuretic peptide receptor in cardiac myocyte caveolae. Circ Res 81:86–91
Koglin M, Behrends S (2004) Native human nitric oxide sensitive guanylyl cyclase: purification and characterization. Biochem Pharmacol 67:1579–1585
Foerster J, Harteneck C, Malkewitz J, Schultz G, Koesling D (1996) A functional heme-binding site of soluble guanylyl cyclase requires intact N-termini of alpha 1 and beta 1 subunits. Eur J Biochem 240:380–386
Balashova N, Chang FJ, Lamothe M, Sun Q, Beuve A (2005) Characterization of a novel type of endogenous activator of soluble guanylyl cyclase. J Biol Chem 280:2186–2196
Russwurm M, Wittau N, Koesling D (2001) Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J Biol Chem 276:44647–44652
Linder AE, McCluskey LP, Cole KR III, Lanning KM, Webb RC (2005) Dynamic association of nitric oxide downstream signaling molecules with endothelial caveolin-1 in rat aorta. J Pharmacol Exp Ther 314:9–15
Linder AE, Leite R, Lauria K, Mills TM, Webb RC (2006) Penile erection requires association of soluble guanylyl cyclase with endothelial caveolin-1 in rat corpus cavernosum. Am J Physiol Regul Integr Comp Physiol 290:R1302–R1308
Venema RC, Venema VJ, Ju H, Harris MB, Snead C, Jilling T, Dimitropoulou C, Maragoudakis ME, Catravas JD (2003) Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am J Physiol Heart Circ Physiol 285:H669–H678
Airhart N, Yang YF, Roberts CT Jr, Silberbach M (2003) Atrial natriuretic peptide induces natriuretic peptide receptor-cGMP-dependent protein kinase interaction. J Biol Chem 278:38693–38698
Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC (1997) Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem 272:25437–25440
Russell KS, Haynes MP, Caulin-Glaser T, Rosneck J, Sessa WC, Bender JR (2000) Estrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells. Effects on calcium sensitivity and NO release. J Biol Chem 275:5026–5030
Dedio J, Konig P, Wohlfart P, Schroeder C, Kummer W, Muller-Esterl W (2001) NOSIP, a novel modulator of endothelial nitric oxide synthase activity. Faseb J 15:79–89
Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V (2001) Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem 276:14249–14256
Potter LR, Garbers DL (1992) Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem 267:14531–14534
Newaz MA, Ranganna K, Oyekan AO (2004) Relationship between PPARalpha activation and NO on proximal tubular Na+ transport in the rat. BMC Pharmacol 4:1
Aperia A, Holtback U, Syren ML, Svensson LB, Fryckstedt J, Greengard P (1994) Activation/deactivation of renal Na+, K(+)-ATPase: a final common pathway for regulation of natriuresis. Faseb J 8:436–439
Beltowski J, Marciniak A, Wojcicka G, Gorny D (2003) Regulation of renal Na(+), K(+)-ATPase and ouabain-sensitive H(+), K(+)-ATPase by the cyclic AMP-protein kinase A signal transduction pathway. Acta Biochim Pol 50:103–114
Elesgaray R, Caniffi C, Ierace DR, Jaime MF, Fellet A, Arranz C, Costa MA (2008) Signaling cascade that mediates endothelial nitric oxide synthase activation induced by atrial natriuretic peptide. Regul Pept 151:130–134
McLay JS, Chatterjee PK, Jardine AG, Hawksworth GM (1995) Atrial natriuretic factor modulates nitric oxide production: an ANF-C receptor-mediated effect. J Hypertens 13:625–630
William M, Hamilton EJ, Garcia A, Bundgaard H, Chia KK, Figtree GA, Rasmussen HH (2008) Natriuretic peptides stimulate the cardiac sodium pump via NPR-C-coupled NOS activation. Am J Physiol Cell Physiol 294:C1067–C1073
Correa AH, Choi MR, Gironacci M, Valera MS, Fernandez BE (2007) Signaling pathways involved in atrial natriuretic factor and dopamine regulation of renal Na+, K+-ATPase activity. Regul Pept 138:26–31
Hakam AC, Hussain T (2006) Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol 290:F1430–F1436
Abraham NG (2008) Gene targeting and heme oxygenase-1 expression in prevention of hypertension induced by angiotensin II. Hypertension 52:618–620
Acknowledgments
Support: National Institute of Health H R21DK065628-02 (to RSD); American Heart Association Grant-in-Aid (to RSD); Tobacco Research Institute (to RSD); National Institute of Health K01 DK071641-03 (to KUK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kotlo, K.U., Rasenick, M.M. & Danziger, R.S. Evidence for cross-talk between atrial natriuretic peptide and nitric oxide receptors. Mol Cell Biochem 338, 183–189 (2010). https://doi.org/10.1007/s11010-009-0352-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0352-6