Abstract
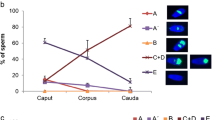
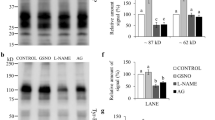
Protein tyrosine phosphorylation is a key event accompanying sperm capacitation. Although this signaling cascade generates an array of tyrosine-phosphorylated polypeptides, their molecular characterization is still limited. It is necessary to differentiate the localization of the tyrosine-phosphorylated proteins in spermatozoa to understand the link between the different phosphorylated proteins and the corresponding regulated sperm function. cAMP plays a pivotal role in the regulation of tyrosine phosphorylation. The intracellular cAMP levels were raised in goat spermatozoa by the addition of the phosphodiesterase inhibitor, IBMX in conjugation with caffeine. Tyrosine phosphorylation was significantly up-regulated following treatment with these two reagents. Treatment of caudal spermatozoa with IBMX and caffeine, time dependent up-regulated phosphorylation of the protein of molecular weights 50 and 200 kDa was observed. Increased phosphorylation was observed with a combination of IBMX and caffeine treatment. Tyrosine phosphorylation in caput spermatozoa was not affected significantly under these conditions. The expression level of tyrosine kinase in sperm was examined with specific inhibitors and with anti-phosphotyrosine antibody. The indirect immunofluorescence staining was carried out on ethanol permeabilized sperm using anti-phosphotyrosine antibody. Western blot analysis was done using two separate PKA antibodies: anti-PKA catalytic and anti-PKA RIα. Almost no difference was found in the intracellular presence of the PKA RIα and RIIα subunits in caput and caudal epididymal spermatozoa. However, the catalytic subunit seemed to be present in higher amount in caudal spermatozoa. The results show that caprine sperm displays an enhancement of phosphorylation in the tyrosine residues of specific proteins under in vitro capacitation conditions.










Similar content being viewed by others
References
Naz RK, Rajesh PB (2004) Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocrinol (Review) 2:74–86
Emiliozzic FP (1997) Protein tyrosine phosphorylation is associated with capacitation of human sperm in vitro but is not sufficient for its completion. Biol Reprod 56:674–679
Aitken RJ, Paterson M, Fisher H, Buckingham DW, Van Duin M (1995) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108:2017–2025
Visconti PE, Johnson LR, Oyaski M, Fornés M, Moss SB, Gerton GL, Kopf GS (1997) Regulation, localization and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol 192:351–363
Stauss CR, Volta TJ, Suarez SS (1995) Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod 53:1280–1285
NagDas SK, Winfrey V, Olson GE (2005) Tyrosine phosphorylation generates multiple isoforms of the mitochondrial capsules protein, phospholipid hydroperoxide glutathione peroxidase (PHGPx), during hamster sperm capacitation. Biol Reprod 72:164–171
Leyton L, LeGuen P, Bunch D, Saling P (1992) Regulation of mouse gamete interaction by a sperm tyrosine kinase. Proc Natl Acad Sci USA 89:692–695
Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB (2002) Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 53:133–150
Harrison DA, Carr DW, Meizel S (2000) Involvement of protein kinase A and a kinase anchoring protein in the progesterone-initiated human sperm acrosome reaction. Biol Reprod 62:811–820
Skalhegg BS, Huang Y, Su T, Idzerda RL, McNight GS, Burton KA (2002) Mutation of the C subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol 16:630–639
Vijayaraghavan S, Olson GE, NagDas S, Winfrey VP, Carr DW (1997) Subcellular localization of the regulatory subunits of cyclic adenosine 3′,5′-monophosphate-dependent protein kinase in bovine spermatozoa. Biol Reprod 57:1517–1523
Mandal AK, Roy K, Sil PC, Yadav SP, Sen PC (2001) Purification and characterization of a 70 kDa inhibitor protein of Na+, K+-ATPase from goat testis cytosol. Mol Cell Biochem 223:7–14
Thérien I, Soubeyrand S, Manjunath P (1997) Major proteins of bovine seminal plasma modulate sperm capacitation by high-density lipoprotein. Biol Reprod 57:1080–1088
Miller DJ, Winer MA, Ax RL (1990) Heparin-binding proteins from seminal plasma bind to bovine spermatozoa and modulate capacitation by heparin. Biol Reprod 42:899–915
Parrish JJ, Susko-Parrish J, Winer MA, First NL (1988) Capacitation of bovine sperm by heparin. Biol Reprod 38:1171–1180
Lewis B, Aitken RJ (2001) Impact of epididymal maturation on the tyrosine phosphorylation patterns exhibited by rat spermatozoa. Biol Reprod 64:1545–1556
Visconti PE, Kopf GS (1998) Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 59:1545–1556
Brandt H, Hoskins DD (1980) A cAMP-dependent phosphorylated motility protein in bovine epididymal sperm. J Biol Chem 255:982–987
Flesch FM, Wijnand E, van de Lest CH, Colenbrander B, van Golde LM, Gadella BM (2001) Capacitation dependent activation of tyrosine phosphorylation generates two sperm head plasma membrane proteins with high primary binding affinity for the zona pellucida. Mol Reprod Dev 60:107–115
Bielfeld P, Faridi A, Zaneveld LJD, Jonge CJ (1994) The zona pellucida-induced acrosome reaction of human spermatozoa is mediated by protein kinase. Fertil Steril 61:536–541
Turner RM, Johnson LR, Haig-Ladewig L, Gerton GL, Moss SB (1998) An X-linked gene encodes a major human sperm fibrous sheath protein, hAKAP82. Genomic organization, protein kinase A-RII binding, and distribution of the precursor in the sperm tail. J Biol Chem 273:32135–32141
Van Haastert PJ, Van Driel R, Jastorff B, Baraniak J, Stec WJ, De Wit RJ (1984) Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem 259:10020–10024
Bajpai M, Asins S, Doncel GF (2003) Effect of tyrosine kinase inhibitor on tyrosine phosphorylation and motility parameters. Arch Androl 49:229–246
Louise H, Aitken RJ (2006) Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci 119:3182–3192
Bajpai M, Dancel GF (2003) Involvement of tyrosine kinase and c AMP–dependent kinase cross-talk in the regulation of human sperm motility. Reproduction 126:183–195
Takagi K, Ogawa K, Tanaka H, Satake T, Watanabe Y, Chijiwa T, Hidaka H (1992) Relaxant effects of various xanthine derivatives: relationship to cyclic nucleotide phosphodiesterase inhibition. Adv Second Messenger Phosphoprotein Res 25:353–362
Botelho LH, Rothermel JD, Coombs RV, Jastorff B (1988) cAMP analog antagonists of cAMP action. Methods Enzymol 159:159–172
Boatman DE, Bavister BD (1984) Stimulation of rhesus monkey sperm capacitation by cyclic nucleotide mediators. J Reprod Fertil 71:357–366
Vandevoort CA, Tollner TL, Overstreet JW (1994) Separate effects of caffeine and dbcAMP on macaque sperm motility and interaction with the zona pellucida. Mol Reprod Dev 37:299–304
Parrish JJ, Susko-Parrish JL, Uguz C, First NL (1994) Difference in the role of cyclic adenosine 3′,5′-monophosphate during capacitation of bovine sperm by heparin or oviduct fluid. Biol Reprod 51:1099–1108
Bookbinder LH, Moy GW, Vacquier VD (1991) In vitro phosphorylation of sea urchin sperm adenylate cyclase by cyclic adenosine monophosphate-dependent protein kinase. Mol Reprod Dev 28:150–157
Acknowledgments
This study was supported by the financial assistance from Council of Scientific and Industrial Research (37/1088/01/EMR) and from Bose Institute. Authors are thankful to Arindam Basu for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, M., Nandi, P., Ghosh, S. et al. Regulation of tyrosine kinase activity during capacitation in goat sperm. Mol Cell Biochem 336, 39–48 (2010). https://doi.org/10.1007/s11010-009-0261-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0261-8