Abstract
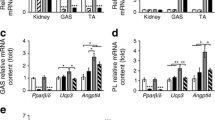
Peroxisome proliferator-activated receptors (PPAR) exist in three different forms, alpha (α), beta/delta (β/δ), or gamma (γ), all of which are expressed in skeletal muscle and play a critical role in the regulation of oxidative metabolism. The purpose of this investigation was to determine the mRNA expression pattern of the different PPARs and peroxisome proliferator-activated receptor alpha coactivator-1 alpha (PGC-1α) in muscles that largely rely on either glycolytic (plantaris) or oxidative (soleus) metabolism. Further, we also examined the alterations in the PPARs mRNA expression after one bout of endurance exercise or after 12 weeks of exercise training in the different muscles. Female Sprague-Dawley rats (5–8 months) were either run on the treadmill once or exercised trained for 12 weeks. The muscles were removed 24 h after the last bout of exercise. The results demonstrated with the exception of PPAR β/δ, the PPAR mRNAs are expressed to a greater extent in the soleus muscle than in the plantaris muscle in sedentary animals. PPARγ was the least abundantly expressed PPAR in either the soleus or the plantaris muscle. With respect to exercise training, only PPARγ mRNA expression increased in the soleus muscle, while PPARβ/δ and γ mRNA levels increased in the plantaris muscle. Minimal changes were detected in any of the PPARs with one bout of exercise training. These results suggest that PPARγ mRNA levels are the lowest in skeletal muscle among all of the PPARs and PPARγ mRNA is the most responsive to changes in physical activity levels.






Similar content being viewed by others
References
Holloszy JO (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242:2278–2282
Hood DA, Irrcher I, Ljubicic V et al (2006) Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209:2265–2275
Muoio DM, Koves TR (2007) Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab 32:874–883
Gilde AJ, Van Bilsen M (2003) Peroxisome proliferator-activated receptors (PPARS): regulators of gene expression in heart and skeletal muscle. Acta Physiol Scand 178:425–434
Calvo JA, Daniels TG, Wang X et al (2008) Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol 104:1304–1312
Brown DA, Lynch JM, Armstrong CJ et al (2005) Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol 564:619–630
Spangenburg EE, Brown DA, Johnson MS et al (2006) Exercise increases SOCS-3 expression in rat skeletal muscle: potential relationship to IL-6 expression. J Physiol 572:839–848
Srere P (1969) Citrate synthase. Methods Enzymol 13:3–5
Spangenburg EE, Abraha T, Childs TE et al (2003) Skeletal muscle IGF-binding protein-3 and -5 expressions are age, muscle, and load dependent. Am J Physiol Endocrinol Metab 284:E340–E350
Spangenburg EE (2005) SOCS-3 induces myoblast differentiation. J Biol Chem 280:10749–10758
Armstrong RB, Phelps RO (1984) Muscle fiber type composition of the rat hindlimb. Am J Anat 171:259–272
Lunde IG, Ekmark M, Rana ZA et al (2007) PPARdelta expression is influenced by muscle activity and induces slow muscle properties in adult rat muscles after somatic gene transfer. J Physiol 582:1277–1287
Wang YX, Zhang CL, Yu RT et al (2004) Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2:e294
Mahoney DJ, Parise G, Melov S et al (2005) Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19:1498–1500
Tunstall RJ, Mehan KA, Wadley GD et al (2002) Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab 283:E66–E72
Helge JW, Bentley D, Schjerling P et al (2007) Four weeks one-leg training and high fat diet does not alter PPARalpha protein or mRNA expression in human skeletal muscle. Eur J Appl Physiol 101:105–114
Muoio DM, MacLean PS, Lang DB et al (2002) Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem 277:26089–26097
Degenhardt T, Saramaki A, Malinen M et al (2007) Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor beta/delta. J Mol Biol 372:341–355
Villarroya F, Iglesias R, Giralt M (2007) PPARs in the control of uncoupling proteins gene expression. PPAR Res 2007:74364
Riquet FB, Rodriguez M, Guigal N et al (2003) In vivo characterisation of the human UCP3 gene minimal promoter in mice tibialis anterior muscles. Biochem Biophys Res Commun 311:583–591
Russell AP, Wadley G, Hesselink MK et al (2003) UCP3 protein expression is lower in type I, IIa and IIx muscle fiber types of endurance-trained compared to untrained subjects. Pflugers Arch 445:563–569
Siu PM, Donley DA, Bryner RW et al (2004) Myogenin and oxidative enzyme gene expression levels are elevated in rat soleus muscles after endurance training. J Appl Physiol 97:277–285
Yan Z, Li P, Akimoto T (2007) Transcriptional control of the PGC-1alpha gene in skeletal muscle in vivo. Exerc Sport Sci Rev 35:97–101
Acknowledgements
The authors wish to thank Emily Pettycrew, Matt Lucero, and Christian Alvarez for their expert technical assistance. These experiments were supported, in part, by National Institutes of Health grants HL 40306-15 (RLM) and HL 72790-02 (RLM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spangenburg, E.E., Brown, D.A., Johnson, M.S. et al. Alterations in peroxisome proliferator-activated receptor mRNA expression in skeletal muscle after acute and repeated bouts of exercise. Mol Cell Biochem 332, 225–231 (2009). https://doi.org/10.1007/s11010-009-0195-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0195-1