Abstract
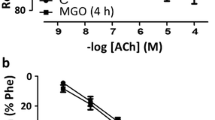
As there are increasing evidences that human diabetes induces cardiovascular dysfunction, we investigated the type-2 diabetes-induced endothelial dysfunction in the early and late-stage Goto-Kakizaki (GK) rat aorta. We performed organ bath studies, and examined the changes in expression levels of muscarinic M3 receptor, endothelial, inducible, and neuronal nitric oxide synthase (eNOS, iNOS, and nNOS, respectively) mRNAs in the rat aorta utilizing real-time polymerase chain reaction in 12-week-old and 70-week-old GK rats as well as in age-matched Wistar rats. In the 12-week-old GK rat aorta, a significant increase in norepinephrine-induced contraction and a significant decrease in acetylcholine-induced relaxation as well as significant increases in expression levels of muscarinic M3 receptor and eNOS and a significant decease in nNOS mRNAs were observed compared to age-matched controls. In the older GK rat aorta, significant decreases in acetylcholine- and nitroglycerine-induced relaxations as well as significant decreases in the expression levels of muscarinic M3 receptor, eNOS, iNOS, and nNOS mRNAs were observed compared to those in the younger GK rats. In contrast, although significant decreases in acetylcholine and nitroglycerine-induced relaxations were observed, the expression levels of muscarinic M3 receptor, eNOS, iNOS, and nNOS mRNAs in the older Wistar rats aorta were unchanged, increased, increased and decreased, respectively, compared to the younger Wistar rat aorta. These results indicate that endothelial dysfunction in the rat aorta progresses with age and development of diabetes condition, and that decreased relaxations in the late-stage rat aorta may be due to these alterations.



Similar content being viewed by others
References
Amos AF, McCarty DJ, Zimmet P (1997) The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetes Med 14:S1–S85
Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA (2006) Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 291:H1780–H1787
Furchgott RF, Vanhoutte PM (1989) Endothelium-derived relaxing and contracting factors. FASEB J 3:2007–2018
Furchgott RF (1999) Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep 1:235–251
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM (2000) Endothelial dysfunction in diabetes. Br J Pharmacol 130:963–974
Feletou M, Vanhoutte PM (1999) The third pathway: endothelium-dependent hyperpolarization. J Physiol Pharmacol 50:525–534
Huszka M, Kaplar M, Rejto L, Tornai I, Palatka K, Laszlo P, Udvardy M (1997) The association of reduced endothelium derived relaxing factor-NO production with endothelial damage and increased in vivo platelet activation in patients with diabetes mellitus. Thromb Res 86:173–180
Boulanger CM, Morrison KJ, Vanhoutte PM (1994) Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol 112:519–524
Khurana S, Chacon I, Xie G, Yamada M, Wess J, Raufman JP, Kennedy RH (2004) Vasodilatory effects of cholinergic agonists are greatly diminished in aorta from M3R−/−mice. Eur J Pharmacol 493:127–132
Goto Y, Kakizaki M, Masaki N (1976) Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119:85–90
Salehi A, Carlberg M, Henningson R, Lundquist I (1996) Islet constitutive nitric oxide synthase: biochemical determination and regulatory function. Am J Physiol 270:C1634–C1641
Unger RH (1976) The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes 25:136–151
Murakawa Y, Zhang W, Pierson CR, Brismar T, Östenson CG, Efendic S, Sima AA (2002) Impaired glucose tolerance and insulinopenia in the GK-rat causes peripheral neuropathy. Diabetes Metab Res Rev 18:473–483
Yagihashi S, Tnosaki A, Yamada K, Kakizaki M, Goto Y (1982) Peripheral neuropathy in selectively-inbred spontaneously diabetic rats: Electrophysiological, morphometrical and freeze-replica studies. Tohoku J Exp Med 138:39–48
Shinbori C, Saito M, Kinoshita Y, Satoh I, Kono T, Hanada T, Nanba E, Adachi K, Suzuki H, Yamada M, Satoh K (2007) Cyclohexenonic long-chain fatty alcohol has therapeutic effects on diabetes-induced angiopathy in the rat aorta. Eur J Pharmacol 56:139–144
Portha B, Lacraz G, Kergoat M, Homo-Delarche F, Giroix MH, Bailbé D, Gangnerau MN, Dolz M, Tourrel-Cuzin C, Movassat J (2009) The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol 297:73–85
Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P (2001) Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metabol 27:436–447
Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:E14–E22
Pannirselvam M, Verma S, Anderson TJ, Triggle CR (2002) Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol 136:255–263
Kobayashi T, Matsumoto T, Ooishi K, Kamata K (2004) Differential expression of alpha2D-adrenoceptor and eNOS in aortas from early and later stages of diabetes in Goto-Kakizaki rats. Am J Physiol Heart Circ Physiol 287:H135–H143
Zhang C, Yang J, Jennings LK (2004) Leukocyte-derived myeloperoxidase amplifies high-glucose-induced endothelial dysfunction through interaction with high-glucose-stimulated, vascular non-leukocyte-derived reactive oxygen species. Diabetes 53:2950–2959
Bitar MS, Wahid S, Mustafa S, AI-Saleh E, Dhaunsi GS, AI-Mulla F (2005) Nitric oxide dynamics and endothelial dysfunction on type II model of genetic diabetes. Eur J Pharmacol 511:53–64
Sandu OA, Ragolia L, Begum N (2000) Diabetes in the Goto-Kakizaki rat is accompanied by impaired insulin-mediated myosin-bound phosphatase activation and vascular smooth muscle cell relaxation. Diabetes 49:2178–2189
Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L, Lemmer B (2002) Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto-Kakizaki rats: influence of age and diabetic state. Nitric Oxide 6:85–95
Chen S, Mukherjee S, Chakraborty C, Chakrabarti (2003) High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-kappa B and AP-1. Am J Physiol Cell Physiol 284:C263–C272
Elgebaly MM, Kelly A, Harris AK, Elewa H, Portik-Dobos V, Ketsawatsomkron P, Marrero M, Ergul A (2008) Impaired insulin-mediated vasorelaxation in a nonobese model of type 2 diabetes: role of endothelin-1. Can J Physiol Pharmacol 2008(86):358–364
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Calles-Escandon J, Cipolla M (2001) Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev 22:36–52
Henningsson R, Alm P, Lindstrom E, Lundquist I (2000) Chronic blockade of NO synthase paradoxically increases islet NO production and modulates islet hormone release. Am J Physiol Endocr Metabol 279:E95–E107
Papapetropoulos A, Rudic RD, Sessa WC (1999) Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc Res 43:509–520
Li H, Forstermann U (2000) Nitric oxide in the pathogenesis of vascular disease. J Pathol 190:244–254
Kibbe M, Billiar T, Tzeng E (1999) Inducible nitric oxide synthase and vascular injury. Cardiovasc Res 43:650–657
Pandolfi A, De Filippis EA (2007) Chronic hyperglycemia and nitric oxide bioavailability play a pivotal role in pro-atherogenic vascular modifications. Genes Nutr 2:195–208
Lee JH, Palasia T, Ragolia L (2009) Impaired insulin-mediated vasorelaxation in diabetic Goto-Kakizaki rats is caused by impaired Akt phosphorylation. Am J Physiol Cell Physiol 296:C327–C338
Matsumoto T, Ishida K, Nakayama N, Kobayashi T, Kamata K (2009) Involvement of NO and MEK/ERK pathway in enhancement of endothelin-1-induced mesenteric artery contraction in later-stage type 2 diabetic Goto-Kakizaki rat. Am J Physiol Heart Circ Physiol 296:H1388–H1397
Oyadomari S, Gotoh T, Aoyagi K, Araki E, Shichiri M, Mori M (2001) Coinduction of endothelial nitric oxide synthase and arginine recycling enzymes in aorta of diabetic rats. Nitric Oxide 5:252–260
Cai S, Khoo J, Mussa S, Alp NJ, Channon KM (2005) Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia 48:1933–1940
Ji K, Minakawa M, Fukui K, Suzuki Y, Fukuda I (2008) Increased superoxide radical with a decrease in vascular endothelial growth factor and inducible nitric oxide synthase level leads to the progression of left ventricular hypertrophy in a pressure-overload rat heart model. Ann Thorac Cardiovasc Surg 14:210–217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazuyama, E., Saito, M., Kinoshita, Y. et al. Endothelial dysfunction in the early- and late-stage type-2 diabetic Goto-Kakizaki rat aorta. Mol Cell Biochem 332, 95–102 (2009). https://doi.org/10.1007/s11010-009-0178-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0178-2