Abstract
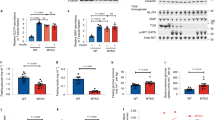
The role of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)- and SNARE-associated proteins have not yet been assessed in regulation of cardiac glucose uptake, nor in the regulation of long-chain fatty acid (LCFA) uptake in any tissue. Munc18c is a SNARE-associated protein that regulates GLUT4 translocation in skeletal muscle and adipose tissue. Using cardiomyocytes from Munc18c−/+ mice (with 56% reduction of Munc18c protein expression), we investigated whether this syntaxin4-associated protein is involved in regulation of cardiac substrate uptake. Basal, insulin- and oligomycin (a 5′ AMP-activated protein kinase-activating agent)-stimulated glucose and LCFA uptake were not altered significantly in Munc18c−/+ cardiomyocytes compared to wild-type cells. We conclude, therefore, that Munc18c is not rate-limiting for cardiac substrate uptake, neither under basal conditions nor when maximally stimulated metabolically.



Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
5′ AMP-activated protein kinase
- CD36:
-
Fatty acid translocase
- GAPDH:
-
Glyceraldehydes-3-phosphate dehydrogenase
- GLUT4:
-
Glucose transporter-4
- LCFA:
-
Long-chain fatty acids
- PKB:
-
Protein kinase-B
- SNAP23:
-
Soluble NSF-attachment protein-23
- t/vSNARE:
-
Target/vesicular soluble N-ethylmaleimide-sensitive factor-attachment protein receptor
- VAMP2:
-
Vesicle-associated membrane protein-2
References
Glatz JF, Bonen A, Ouwens DM et al (2006) Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc Drugs Ther 20:471–476
Luiken JJ, Koonen DP, Willems J et al (2002) Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51:3113–3119
Luiken JJ, Coort SL, Willems J et al (2003) Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52:1627–1634
Watson RT, Pessin JE (2007) GLUT4 translocation: the last 200 nanometers. Cell Signal 19:2209–2217
Rudich A, Klip A (2003) Push/pull mechanisms of GLUT4 traffic in muscle cells. Acta Physiol Scand 178:297–308
Peters CG, Miller DF, Giovannucci DR (2006) Identification, localization and interaction of SNARE proteins in atrial cardiac myocytes. J Mol Cell Cardiol 40:361–374
Ishiki M, Klip A (2005) Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology 146:5071–5078
Hata Y, Slaughter CA, Sudhof TC (1993) Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366:347–351
Thurmond DC, Ceresa BP, Okada S et al (1998) Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J Biol Chem 273:33876–33883
Oh E, Spurlin BA, Pessin JE et al (2005) Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes 54:638–647
Kanda H, Tamori Y, Shinoda H et al (2005) Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest 115:291–301
Habets DD, Coumans WA, Voshol PJ et al (2007) AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun 355:204–210
van Oort MM, van Doorn JM, Bonen A et al (2008) Insulin-induced translocation of CD36 to the plasma membrane is reversible and shows similarity to that of GLUT4. Biochim Biophys Acta 1781:61–71
Luiken JJ, van Nieuwenhoven FA, America G et al (1997) Uptake and metabolism of palmitate by isolated cardiac myocytes from adult rats: involvement of sarcolemmal proteins. J Lipid Res 38:745–758
Luiken JJ, Coort SL, Koonen DP et al (2004) Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch 448:1–15
Tomas E, Sevilla L, Palacin M et al (2001) The insulin-sensitive GLUT4 storage compartment is a postendocytic and heterogeneous population recruited by acute exercise. Biochem Biophys Res Commun 284:490–495
Thurmond DC, Pessin JE (2001) Molecular machinery involved in the insulin-regulated fusion of GLUT4-containing vesicles with the plasma membrane (review). Mol Membr Biol 18:237–245
Zorzano A, Sevilla L, Camps M et al (1997) Regulation of glucose transport, and glucose transporters expression and trafficking in the heart: studies in cardiac myocytes. Am J Cardiol 80:65A–76A
Martin S, Slot JW, James DE (1999) GLUT4 trafficking in insulin-sensitive cells. A morphological review. Cell Biochem Biophys 30:89–113
St-Denis JF, Cushman SW (1998) Role of SNARE’s in the GLUT4 translocation response to insulin in adipose cells and muscle. J Basic Clin Physiol Pharmacol 9:153–165
Oh E, Thurmond DC (2006) The stimulus-induced tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis. J Biol Chem 281:17624–17634
Acknowledgments
This study was supported by the Netherlands Organisation for Health Research and Development (ZonMw grant nr. 40-00812-98-03075), the European Community (Integrated Project LSHM-CT-2004-005272, Exgenesis), the National Institutes of Health (DK67912 to D.C.T.), and the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Heart and Stroke Foundation of Ontario, the Canada Research Chair program. A. Bonen is the Canada Research Chair in Metabolism and Health. J. F. C. Glatz is Netherlands Heart Foundation Professor of Cardiac Metabolism.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habets, D.D.J., Thurmond, D.C., Coumans, W.A. et al. Munc18c is not rate-limiting for glucose and long-chain fatty acid uptake in the heart. Mol Cell Biochem 322, 81–86 (2009). https://doi.org/10.1007/s11010-008-9942-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9942-y