Abstract
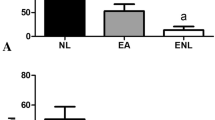
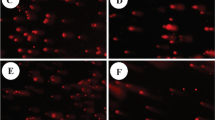
The effectiveness of gelonin to arrest protein synthesis, thereby limiting the growth of cancer cells was studied by encapsulating it into liposomes. The protein was extracted from the seeds of Indian plant Gelonium multiflorum by ammonium sulfate precipitation and purified using cation-exchange and gel-filtration chromatography. Biological activity of purified gelonin was determined using a rabbit reticulocyte lysate assay in the cell-free translational experiments. Gelonin was encapsulated in conventional liposomes prepared by the dry film method in order to retain biological activity of the entrapped protein. Carcinogenesis was induced in Swiss albino mice by intravenous administration of DBN (10 mg kg−1 body weight) at weekly intervals. Marker enzyme assays (GGT, AChE, and GST), GSH levels, cell proliferation assay, hepatocyte DNA analysis, histological examination of micro sections of liver tissues were parameters used to monitor carcinogenesis induction, and regression in mice. From the in vitro experiments conducted, it was observed that gelonin upon its encapsulation into liposome, resulted in significant destruction of the transformed liver cells by its cytotoxic effects that arrest protein synthesis. Various parameters studied to monitor regression also suggested mass cell destruction to liver upon administration of liposomal gelonin in mice exposed to DBN.






Similar content being viewed by others
Abbreviations
- DBN:
-
N,N nitrosodibutylamine
- GGT:
-
γ-Glutamyl transpeptidase
- AchE:
-
Acetylcholine esterase
- GSH:
-
Glutathione (reduced)
- GST:
-
Glutathione-S-transferase
- RIP:
-
Ribosome-inactivating protein
- ED:
-
Effective dose
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- RES:
-
Reticulo-endothelial system
- FCS:
-
Fetal Calf Serum
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- DPPC:
-
Dipalmitoylphosphatidylcholine
- Chol:
-
Cholesterol
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidyl ethanolamine
- PS:
-
Phosphatidyl serine
- PG:
-
Phosphatidyl glycerol
- PEG:
-
Polyethylene glycol
- BrdU:
-
Bromodeoxyuridine
References
Barbeieri L, Battelli MG, Stripe F (1993) Ribosome-inactivating proteins from plants. Biochim Biophys Acta 1154:237–282
Nicolas E, Beggs JM, Haltiwanger BM, Taraschi TF (1997) Direct evidence for the deoxyribonuclease activity of the plant ribosome inactivating protein gelonin. FEBS Lett 406:162–164. doi:10.1016/S0014-5793(97)00267-6
Gao W, Ling J, Zhong X, Liu W, Zhang R, Yang H et al (1994) Luffin S–a small novel ribosome-inactivating protein from Luffa cylindrical. Characterization and mechanism studies. FEBS Lett 347:257–260. doi:10.1016/0014-5793(94)00554-0
Dallal JA, Ivin JD (1978) Enzymatic inactivation of eukaryotic ribosomes by the pokeweed antiviral protein. FEBS Lett 89:257–259. doi:10.1016/0014-5793(78)80230-0
Barbeiri L, Stripe F (1982) Ribosome-inactivating proteins from plants: properties and possible uses. Cancer Surv 1:489–520
Jiminez A, Andvasquez D (1985) Plant and fungal protein and glycoprotein toxins inhibiting eukaryote protein synthesis. Annu Rev Microbiol 39:649–672
Lord JM, Hartely MR, Roberts LM (1991) Ribosome inactivating proteins of plants. Semin Cell Biol 2:15–22
Endo Y, Tsurugi K (1987) RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem 262:8128–8130
Endo Y (1988) Immunotoxins. In: Frankel AE (ed) Kluwer, Boston, p 75
Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes: the site and the characteristics of the modification in 28S ribosomal RNA caused by toxins. J Biol Chem 262:5908–5912
Atkinson SF, Bettinger T, Seymour LW, Behr JF, Ward CM (2001) Conjugation of folate via gelonin carbohydrate residues retains ribosomal-inactivating properties of the toxin and permits targeting to folate receptor positive cells. J Biol Chem 276:27930–27935. doi:10.1074/jbc.M102825200
Better M (1996) T-cell-targeted immunofusion proteins from E. coli. Ann N Y Acad Sci 782:544–554
Lambert JM, Senter PD, Yau-Young A, Battler WA, Goldmacher VS (1985) Purified immunotoxins that are reactive with human lymphoid cells. Monoclonal antibodies conjugated to the ribosome-inactivating proteins gelonin and the pokeweed antiviral proteins. J Biol Chem 260:12035–12041
Singh V, Sairam MR (1989) Hormonotoxins: conjugation of human choriogonadotropin with the ribosome inactivating protein gelonin and comparison with lutropin conjugates. Mol Cell Endocrinol 67:217–229. doi:10.1016/0303-7207(89)90212-8
Singh V, Sairam MR, Bhagavai GN, Akharas RG (1989) Hormonotoxins preparation and characterization of Ovine leutinizing hormone-gelonin conjugates. J Biol Chem 264:3084–3095
Alam A, Bhuri SRK, Mavila AK, Singh V (1992) Design of liposome to improve encapsulation efficiency of gelonin and its effect on immunoreactivity and ribosome inactivating property. Mol Cell Biochem 112:97–107. doi:10.1007/BF00227566
Provoda CJ, Stier EM, Lee KD (2003) Tumor cell killing by listeriolysin O-liposome-mediated delivery of the protein toxin gelonin. J Biochem 278:35102–35108
Stripe F, Olsnes S, Pihl A (1980) Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem 255:6947–6953
Singh V, Singh RC, Dubey RK, Alam A (1999) Purification and characterization of gelonin from sees of Gelonium multiflorum. Indian J Biochem Biophys 36:258–265
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:12863
Kirby CJ, Gregoriadis G (1984) A simple procedure for preparing liposomes capable of high encapsulation efficiency under mild conditions. In Gregoriadis G (ed) Liposome technology, vol 1. CRC Press, Boca Raton, pp 19–35
Meister A, Tate SS, Griffith OW (1981) γ-Glutamyl transpeptidase. Methods Enzymol 16:307
Ellman GL, Courtney DK, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Habig WH, Jakoby WB (1981) Glutathione-S-transferases (rat and human). Methods Enzymol 77:218
Ellman GL (1959) Tissue sulfhydryl group. Arch Biochem Biophys 82:70
Ratcliffe NA (1983) Practical illustrated histology. The Macmillan Press, pp 32–24
Fry JR (1981) Preparation of mammalian hepatocytes. Methods Enzymol 77:130
Seglen PO (1994) Isolation of hepatocytes. In: Cell biology: a laboratory handbook, Academic Press Inc., p 96
Alam A, Singha LI, Singh V (2005) Molecular characterization of tumour associated antigen in mice exposed to a hepatocarcinogen. Mol Cell Biochem 271:177–188
Gratzner GH (1982) Monoclonal antibodies to 5-bromo and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science 218:474–475
Blin N, Stafford DW (1976) A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res 3:2303–2308
Singh V, Kar SK (1992) Properties of a ribosome-inactivating protein, gelonin, purified using three different methods. Indian J Biochem Biophys 29(1):31–41
Papahadjopoulos D (ed) (1978) Liposomes and their uses in biology and medicine. Ann N Y Acad Sci 308
Vertut-Doi, Ishiwata H, Miyajima K (1996) Binding and uptake of liposomes containing poly (ethylene glycol) derivative of cholesterol (stealth liposomes) by a macrophage cell line J774: influence of PEG content and its molecular weight. Biochem Biophys Acta 1278:19
Ying TS, Sarma DSR, FarberE (1982) Effects of the delays in the cell cycle on the initiation of preneoplastic lesions in rat liver by 1,2-dimethylhydrazine. Cancer Res 42:876
Naoyuki T (1974) Purification and some properties of γ-glutamyl transpeptidase from azodye-induced hepatoma. J Biochim 75:473
Fiala S, Reuber MD (1970) Gann High glutathiome activity in “minimum deviation” Reuber hepatoma H-139 61:275
Kosower ES, Kosower EM (1978) The glutathione status of cells. Int Rev Cytosol 54:109
Columbano A, Rajalakhsmi S, Sarma DSR (1980) Requirement of cell proliferation for the initiation of liver carcinogenesis as assayed by three different procedures. Cancer Res 41:2079
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, A., Nakhuru, K.S. & Singha, L.I. Carcinogenesis response modulation induced by gelonin encapsulated in liposome. Mol Cell Biochem 315, 85–95 (2008). https://doi.org/10.1007/s11010-008-9792-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9792-7