Abstract
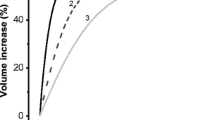
Cell swelling, regulatory volume decrease (RVD), volume-sensitive Cl− (Cl− swell) current and taurine efflux after exposure to high concentrations of urea were characterized in fibroblasts Swiss 3T3, and results compared to those elicited by hyposmotic (30%) swelling. Urea 70, 100, and 150 mM linearly increased cell volume (8.25%, 10.6%, and 15.7%), by a phloretin-inhibitable process. This was followed by RVD by which cells exposed to 70, 100, or 150 mM urea recovered 27.6%, 38.95, and 74.1% of their original volume, respectively. Hyposmolarity (30%) led to a volume increase of 25.9% and recovered volume in 32.5%. 3H-taurine efflux was increased by urea with a sigmoid pattern, as 9.5%, 18.9%, 71.5%, and 89% of the labeled taurine pool was released by 70, 100, 150, or 200 mM urea, respectively. Only about 11% of taurine was released by 30% hyposmolarity reduction in spite of the high increase in cell volume. Urea-induced taurine efflux was suppressed by NPPB (100 μM) and markedly reduced by the tyrosine kinase-general blocker AG18. The Cl− swell current was more rapidly activated and higher in amplitude in the hyposmotic than in the isosmotic/urea condition (urea 150 mM), but this was not sufficient to accomplish an efficient RVD. These results showed that at similar volume increase, cells swollen by urea showed higher taurine efflux, lower Cl− swell current and more efficient RVD, than in those swollen by hyposmolarity. The correlation found between RVD efficiency and taurine efflux suggest a prominent role for organic over ionic osmolytes for RVD evoked by urea in isosmotic conditions.








Similar content being viewed by others
References
Lang F, Busch GL, Volkl H (1998) The diversity of volume regulatory mechanisms. Cell Physiol Biochem 8:1–45
Pasantes-Morales H, Lezama RA, Ramos-Mandujano G et al (2006) Mechanisms of cell volume regulation in hypo-osmolality. Am J Med 119:S4–S11
Hoffmann EK, Pedersen SF (2006) Sensors and signal transduction pathways in vertebrate cell volume regulation. Contrib Nephrol 152:54–104
Pasantes-Morales H, Cardin V, Tuz K (2000) Signaling events during swelling and regulatory volume decrease. Neurochem Res 25:1301–1314
Pasantes-Morales H, Franco R (2005) Astrocyte cellular swelling: mechanisms and relevance to brain edema. In: Aschner M, Costa L (eds) The role of Glia in neurotoxicity, 2nd edn. CRC Press, Boca Raton, FL
Bagnasco SM (2005) Role and regulation of urea transporters. Pflugers Arch 450:217–226
Sands JM (2003) Mammalian urea transporters. Annu Rev Physiol 65:543–566
Shayakul C, Hediger MA (2004) The SLC14 gene family of urea transporters. Pflugers Arch 447:603–609
Ogami A, Miyazaki H, Niisato N et al (2006) UT-B1 urea transporter plays a noble role as active water transporter in C6 glial cells. Biochem Biophys Res Commun 351:619–624
Shennan DB, Grant AC, Gow IF (2002) The effect of hyposmotic and isosmotic cell swelling on the intracellular [Ca2+] in lactating rat mammary acinar cells. Mol Cell Biochem 233:91–97
Jackson PS, Strange K (1993) Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol 265:C1489–C1500
Sanchez-Olea R, Peña C, Moran J et al (1993) Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl− transport in cultured astrocytes. Neurosci Lett 156:141–144
Junankar PR, Kirk K (2000) Organic osmolyte channels: a comparative view. Cell Physiol Biochem 10:355–360
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am J Physiol 273:C755–C789
Cardin V, Pena-Segura C, Pasantes-Morales H (1999) Activation and inactivation of taurine efflux in hyposmotic and isosmotic swelling in cortical astrocytes: role of ionic strength and cell volume decrease. J Neurosci Res 56:659–667
Cannon CL, Basavappa S, Strange K (1998) Intracellular ionic strength regulates the volume sensitivity of a swelling-activated anion channel. Am J Physiol 275:C416–C422
Guizouarn H, Motais R (1999) Swelling activation of transport pathways in erythrocytes: effects of Cl−, ionic strength, and volume changes. Am J Physiol 276:C210–C220
Wittels KA, Hubert EM, Musch MW, Goldstein L (2000) Osmolyte channel regulation by ionic strength in skate RBC. Am J Physiol Regul Integr Comp Physiol 279:R69–R76
Pasantes-Morales H, Lezama RA, Ramos-Mandujano G (2006) Tyrosine kinases and osmolyte fluxes during hyposmotic swelling. Acta Physiol (Oxf) 187:93–102
Cohen DM (1999) Signalling and gene regulation by urea and NaCl in the renal medulla. Clin Exp Pharmacol Physiol 26:69–73
Xu H, Tian W, Lindsey JN et al (2005) EphA2: expression in the renal medulla and regulation by hypertonicity and urea stress in vitro and in vivo. Am J Physiol Renal Physiol 288:F855–F866
Zhang Z, Yang XY, Soltoff SP et al (2000) PI3K signalling in the murine kidney inner medullary cell response to urea. Am J Physiol Renal Physiol 278:F155–F164
Pasantes-Morales H, Moran J, Schousboe A (1990) Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. GLIA 3:427–432
Nilius B, Eggermont J, Voets T et al (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Okada Y (2006) Cell volume-sensitive chloride channels: phenotypic properties and molecular identity. Contrib Nephrol 152:9–24
Zhang J, Lieberman M (1996) Cloride conductance is activated by membrane distension of cultured chick heart cells. Cardiovasc Res 32:168–179
Sabirov RZ, Prenen J, Tomita T, Droogmans G, Nilius B (2000) Reduction of ionic strength activates single volume-regulated anion channels (VRAC) in endothelial cells. Pflugers Arch 439:315–320
Cardin V, Lezama R, Torres-Marquez ME, Pasantes-Morales H (2003) Potentiation of the osmosensitive taurine release and cell volume regulation by cytosolic Ca2+ rise in cultured cerebellar astrocytes. Glia 44:119–128
Ordaz B, Tuz K, Ochoa LD, Lezama R, Pena-Segura C, Franco R (2004) Osmolytes and mechanisms involved in regulatory volume decrease under conditions of sudden or gradual osmolarity decrease. Neurochem Res 29:65–72
Acknowledgments
We deeply acknowledge the valuable technical assistance of Ms. Claudia Peña-Segura. This work was supported in part by grants No. 209507 from DGAPA, UNAM, and 46465 from CONACYT, México.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Domínguez, A., Ramos-Mandujano, G., Vázquez-Juárez, E. et al. Regulatory volume decrease after swelling induced by urea in fibroblasts: prominent role of organic osmolytes. Mol Cell Biochem 306, 95–104 (2007). https://doi.org/10.1007/s11010-007-9558-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9558-7