Abstract
Introduction
This study aimed to summarize the evidence describing the relationship between maternal factors during gestation and risk of congenital heart disease (CHD) in offspring.
Methods
PubMed, EMBASE, and the Cochrane Library were searched for potentially relevant reports from inception to May 2021. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) calculated by the random-effects model were used to evaluate the association between maternal factors and CHD risk.
Results
There was a significant association between CHD risk and obesity in pregnancy (OR 1.29, 95% CI 1.22–1.37; P < 0.001), smoking in pregnancy (OR 1.16, 95% CI 1.07–1.25; P < 0.001), maternal diabetes (OR 2.65, 95% CI 2.20–3.19; P < 0.001), and exposure of pregnant women to organic solvents (OR 1.82, 95% CI 1.23–2.70; P = 0.003). No correlations were revealed between CHD susceptibility and advanced maternal age (OR 1.04, 95% CI 0.96–1.12; P = 0.328), underweight (OR 1.02, 95% CI 0.96–1.08; P = 0.519), alcohol intake in pregnancy (OR 1.08, 95% CI 0.95–1.22; P = 0.251), coffee intake (OR 1.18, 95% CI 0.97–1.44; P = 0.105), and exposure to irradiation (OR 1.80, 95% CI 0.85–3.80; P = 0.125).
Discussion
Maternal factors including maternal obesity, smoking in pregnancy, maternal diabetes and exposure to organic solvents might predispose the offspring to CHD risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Significance
What is already known on this subject? Several maternal factors including overweight and obesity were associated with CHD progression in children. Maternal underweight was found not to be associated with increased susceptibility to CHD in offspring.
What this study adds? Other maternal factors including smoking, diabetes and exposure to organic solvents were significantly associated with an elevated risk of CHD in children. In addition, maternal alcohol or coffee intake, and exposure to irradiation showed no associations with CHD risk in offspring. Furthermore, these associations could be influenced by study design, reported outcomes and models adopted to adjust for confounders.
Introduction
CHD represents the most common malformation diagnosed in newborns globally, and is mainly characterized by incomplete cardiac development from 1 to 6 weeks of pregnancy. The prevalence of CHD was found to be nearly 4–5/1000 live births, with the highest rate reported in Asia (9.3/1000 live births) and the lowest rate in Africa (1.9/1000 live births) (Hoffman & Kaplan, 2002; van der Linde et al., 2011). Moreover, with the passage of time, the prevalence of CHD showed an “S”-shaped graph, with 0.6/1000 and 9.1/1000 live births recorded in 1930 and 2011, respectively (van der Linde et al., 2011). Most patients are diagnosed with severe CHD through cardiac catheterization. Although several medical and surgical strategies have been developed to improve the survival rate of infants with CHD, not all neonatal patients can be successfully treated with surgery. The treatment effects are related to social, economic and personal factors (Kirklin et al., 1990, 1992; Murphy et al., 1993). Moreover, the long-term prognosis of CHD infants undergoing surgeries is not clearly determined (Pacifico et al., 1990). Therefore, the importance and potential impact of maternal factors on primary prevention of CHD disease in offspring should be determined.
Multiple systematic reviews and meta-analyses have been performed to assess the influence of maternal factors on CHD progression in offspring. Sun et al. meta-analyzed 19 case–control and 4 cohort studies, and the results demonstrated that maternal alcohol intake was not significantly associated with CHD in offspring (Sun et al., 2015). Hoang et al. reported that pre-gestational diabetes had a significant linkage with all CHD phenotypes (Hoang et al., 2017). Zhu et al. meta-analyzed 13 case–control and 4 cohort studies, and found that maternal overweight and obesity, rather than maternal underweight, were associated with increased susceptibility to CHD in offspring (Zhu et al., 2018). However, several other maternal factors potentially influencing CHD risk in offspring, including maternal age, smoking history, coffee intake, irradiation and exposure to organic solvents, were not addressed in the aforementioned studies. Therefore, this meta-analysis aimed to comprehensively examine the available reports to clarify the relationship between multiple maternal-associated factors and CHD risk in children.
Methods
Search Strategy and Study Selection
The current systematic review and meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) guideline. PubMed, EMBASE, and the Cochrane Library were systematically queried for potentially relevant reports from inception to May 2021, with “maternal” AND “congenital heart disease” AND “infant” OR “newborn” OR “offspring” as core search terms. Observational studies evaluating the associations between maternal factors and CHD risk in offspring were eligible for screening, with no restrictions on language and publication status. Then, a thorough review of the reference lists of relevant reports was performed to manually identify additional eligible studies.
Based on eligibility criteria, two investigators carried out the search in an independent manner, and a third investigator was involved in case of a disagreement between them. The inclusion criteria were: (1) all participants being pregnant women, with the number of CHD cases in children reported; (2) two or more studies investigating the same maternal factors including age, body mass index (BMI), alcohol intake, smoking history, diabetes, coffee intake, irradiation, and exposure to organic solvents; (3) outcomes including the risk of CHD, and atrial (ASD) and ventricular (VSD) septal defects in children; (4) reporting effect estimates and corresponding 95% confidence intervals (CIs) or raw data from which the effect estimates and 95% CIs could be calculated and combined. The exclusion criteria were: (1) review; (2) animal studies; (3) studies not reporting the estimates of the influence of maternal factors on offspring CHD. All included studies in this meta-analysis were approved by the appropriate ethics committee and were performed in accordance with the 1964 Declaration of Helsinki and its later amendments. All patients gave their informed consents prior to enrollment in all included prospective studies.
Data Collection and Study Quality Evaluation
The data collected included the first author’s surname, publication year, trial design, country, CHD case and non-CHD case numbers, maternal factors, outcomes, and confounding factors. The quality of retrieved studies were assessed using the Newcastle–Ottawa Scale (NOS), which encompassed four selection, one comparability and three outcome subscales. A star rating system ranging between 0 and 9 was employed for scoring observational studies (Wells et al., 2009). Study quality was independently assessed by two authors, and any discrepancies were adjudicated by a third investigator.
Statistical Analysis
The associations between maternal factors and the risk of CHD in offspring were assigned as binary data, and effect estimates with 95% CIs were obtained from each individual study. Summary results from individual studies of maternal factors reported in multiple categories were assessed by the fixed-effects model. Then, a random-effects model was used for summarizing pooled odds ratios (ORs) and 95% CIs to determine whether there was an association between maternal factor and the risk of CHD in offspring (Ades et al., 2005; DerSimonian & Laird, 1986). Heterogeneity across included trials was evaluated by the I2 and Q statistics, with I2 > 50.0% or P < 0.10 indicating significant heterogeneity (Deeks et al., 2008; Higgins et al., 2003). The robustness of the overall conclusions was assessed by a sensitivity analysis that sequentially excluded individual studies (Tobias, 1999). Subgroup analysis with more than five studies was performed based on study design, reported outcomes and adjustment model. Publication bias was assessed through qualitative (funnel plot) and quantitative [Egger’s and Begg’s tests (Begg & Mazumdar, 1994; Egger et al., 1997)] measures. Two-sided P < 0.05 indicated statistical significance. Statistical analysis was performed with Stata 10.0 (Stata Corporation, USA).
Results
Included Studies
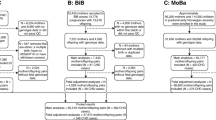
The initial literature search yielded 1146 hits, of which 482 were excluded due to duplication. Then, 559 reports were further excluded due to irrelevance. The remaining 105 reports underwent full-text evaluation, and 41 were excluded for not reporting desirable outcomes (n = 21), assessing paternal factors (n = 13) and being a review or meta-analysis (n = 7). Finally, 64 observational studies were included (Bassili et al., 2000; Bean et al., 2011; Bell et al., 2012; Botto et al., 2000; Carmichael et al., 2003; Cedergren et al., 2002; Correa et al., 2012; Cresci et al., 2011; Eidem et al., 2010; Erickson, 1991; Ewing et al., 1997; Fixler & Threlkeld, 1998; Gilboa et al., 2010; Grewal et al., 2008; Hobbs et al., 2010; Janssen et al., 1996; Källén, 1999; Karatza et al., 2011; Kuciene & Dulskiene, 2009; Loffredo et al., 2001; Malik et al., 2008; Martínez-Frías et al., 2004; Martínez-Frías et al., 2005; Mateja et al., 2012; McDonald et al., 1992; Mills et al., 2010; Nielsen et al., 2005; Oddy et al., 2009; Peticca et al., 2009; Rankin et al., 2010; Sharpe et al., 2005; Shaw & Carmichael, 2008; Sheffield et al., 2002; Smedts et al., 2009; Smedts et al., 2012; Steinberger et al., 2002; Strandberg-Larsen et al., 2011; Tikkanen & Heinonen, 1991; Torfs & Christianson, 1999; van Beynum et al., 2010; van Driel et al., 2008; Waller et al., 2007; Wasserman et al., 1996; Watkins & Botto, 2001; Watkins et al., 2003; Williams et al., 2004; Woods & Raju, 2001). Reviewing their reference lists yielded 133 studies, all of which were enclosed in the initial electronic search results. The detailed study selection process was presented in Fig. 1.
Study Characteristics
Of the 64 included studies, 46 were designed as case–control trials, while the remaining 18 adopted a cohort design. These studies assessed a total of 182,290 CHD cases in offspring. The baseline features of the included studies were presented in Table 1. Twenty-nine studies were carried out in the USA, 24 were conducted in European countries, and the remaining 11were performed in Canada, Australia, China, Egypt and Iran. Data on maternal factors influencing the risks of child ASD and VSD were available in 15 and 20 studies, respectively. Based on NOS evaluation, the rating system assigned 8 stars to 11 studies, 7 stars to 23 studies, 6 stars to 17 studies, and 5 stars to the remaining studies.
Maternal Age
Nineteen studies assessed the influence of maternal age on CHD risk in offspring. This parameter was not significantly associated with subsequent risk of CHD in children (OR 1.04, 95% CI 0.96–1.12; P = 0.328; Fig. 2). Significant heterogeneity among trials was observed (I2 = 74.3%; P < 0.001). The above conclusion was unaltered after sequential exclusion of individual studies (Supplemental 1). Subgroup analysis revealed no significant correlation of maternal age with CHD risk in various subsets. After adjustment for potential confounding factors, advanced maternal age was shown to be associated with increased CHD risk in offspring (OR 1.09, 95% CI 1.00–1.19; P = 0.041; Table 2). No significant publication bias (PEgger, = 0.092, PBegg = 0.624; Supplemental 2) was revealed.
Maternal BMI
Twenty-three studies assessed the influence of maternal obesity on offspring CHD risk. Maternal obesity was shown to be associated with elevated CHD risk (OR 1.29, 95% CI 1.22–1.37; P < 0.001; Fig. 3). There was moderate heterogeneity among trials (I2 = 47.0%; P = 0.007). According to sensitivity analysis, the conclusion remained unaltered after excluding individual studies (Supplemental 1). In subgroup analysis, maternal obesity was correlated with elevated CHD risk but not VSD risk (Table 2). There was no significant publication bias (PEgger, = 0.143, PBegg = 0.958; Supplemental 2).
There were 20 reports assessing the relationship between maternal underweight and the incidence of CHD in offspring. Maternal underweight was not significantly linked with CHD risk (OR 1.02, 95% CI 0.96–1.08; P = 0.519; Fig. 3). No significant heterogeneity across the studies was found (I2 = 0.0%; P = 0.672). The conclusion remained stable after exclusion of any given trial (Supplemental 1). In subgroup analysis, all subsets had findings consistent with the overall analysis, suggesting no significant correlation of maternal underweight with CHD risk (Table 2). There was no publication bias (PEgger = 0.766, PBegg = 0.871; Supplemental 2).
Maternal Alcohol Intake
The pooled analysis of 29 studies (32 cohorts) evaluating maternal alcohol intake suggested that this parameter was not significantly associated with CHD risk in offspring (OR 1.08, 95% CI 0.95–1.22; P = 0.251; Fig. 4). Although significant heterogeneity was detected among trials (I2 = 86.2%; P < 0.001), this conclusion remained unchanged after individual studies were sequentially excluded (Supplemental 1). Subgroup analysis showed consistent findings in various subsets. However, maternal alcohol intake might be linked with an increased risk of CHD when pooling only cohort trials (OR 1.31, 95% CI 0.99–1.72; P = 0.055; Table 2). No significant publication bias was detected (PEgger = 0.053, PBegg = 0.105; Supplemental 2).
Maternal Smoking
The effect of maternal smoking was assessed in 32 studies (33 cohorts). The pooled results showed a significant association between maternal smoking and the development of CHD in offspring (OR 1.16, 95% CI 1.07–1.25; P < 0.001; Fig. 5). Despite significant heterogeneity across studies (I2 = 71.0%; P < 0.001), the above conclusion remained unaffected by the exclusion of any particular study (Supplemental 1). In subgroup analysis, significantly increased risk was detected mainly by pooling case–control studies (OR 1.17, 95% CI 1.06–1.29; P = 0.001), studies reporting the VSD outcome (OR 1.26, 95% CI 1.03–1.54; P = 0.023), and studies adjusting for potential confounding factors (OR 1.16, 95% CI 1.06–1.27; P = 0.001). There was no significant publication bias (PEgger = 0.248, PBegg = 0.710; Supplemental 2).
Maternal Diabetes
The pooled results of 24 studies (26 cohorts) suggested that maternal diabetes had a significant correlation with elevated CHD risk in offspring (OR 2.65, 95% CI 2.20–3.19; P < 0.001; Fig. 6). There was significant heterogeneity among trials (I2 = 92.9%, P < 0.001). The above conclusion was robust and unaltered upon sequential exclusion of individual studies (Supplemental 1). Subgroup analysis revealed that maternal diabetes was related to increased risk of CHD in various subsets (Table 2). There was potential significant publication bias (PEgger = 0.039, PBegg = 0.378; Supplemental 2).
Coffee, Irradiation, and Exposure to Organic Solvents
A total of three (four cohorts), two (three cohorts), and five (six cohorts) studies respectively evaluated the correlations of coffee consumption, irradiation and exposure to organic solvents with CHD risk in offspring. Exposure of pregnant women to organic solvents showed a significant association with elevated CHD risk (OR 1.82, 95% CI 1.23–2.70; P = 0.003), but not with maternal coffee consumption (OR 1.18, 95%CI 0.97–1.44; P = 0.105) and exposure to irradiation (OR 1.80, 95% CI 0.85–3.80; P = 0.125) (Fig. 7). There was significant heterogeneity among studies assessing exposure to organic solvents (I2 = 74.6%; P = 0.001). A moderate heterogeneity was detected across studies on irradiation (I2 = 36.0%; P = 0.210), and no evidence of heterogeneity was found among studies on maternal coffee intake (I2 = 0.0%; P = 0.549).
Discussion
This meta-analysis focusing on observational trials evaluated the potential effects of maternal factors on CHD risk in offspring. A total of 182,290 CHD cases in offspring from 64 studies with various individual features were covered for a systematic review. As shown above, maternal obesity, smoking, diabetes and exposure to organic solvents were significantly associated with elevated CHD risk in children. Meanwhile, the correlations of maternal age, underweight in pregnancy, alcohol or coffee intake, and exposure to irradiation with CHD risk in offspring were not consistent. Furthermore, the associations between maternal factors and child CHD could be influenced by study design, reported outcomes and confounder-adjusted models.
The present meta-analysis demonstrated that maternal age was not associated with CHD risk in offspring. However, advanced maternal age could be linked with increased incidence of CHD in children after adjustment for certain confounding factors. It should be noted that many studies reported inconsistent data. Malik and colleagues reported that maternal age ≥ 35.0 (versus < 20.0 years) was associated with elevated CHD risk (Malik et al., 2008). In addition, Liu et al. indicated that maternal age ≥ 35.0 (versus 25.0–29.0 years) was related to an increased CHD risk in offspring (Liu et al., 2013). Furthermore, another study revealed an association between maternal age ≥ 36.0 (versus 15.0–29.0 years) and elevated CHD risk in children (Liu et al., 2018). This might be attributed to chromosomal abnormalities, such as trisomy 21 caused by meiotic nondisjunction errors as oocyte aged. Moreover, advanced maternal age could be linked with increased risk of multiple pregnancy-related complications, including spontaneous abortion, preeclampsia, gestational diabetes, fetal growth restriction and stillbirth (Cleary-Goldman et al., 2005; Jacobsson et al., 2004; Laopaiboon et al., 2014; Salem Yaniv et al., 2011; Stillbirth Collaborative Research Network Writing Group, 2011).
This meta-analysis demonstrated that maternal obesity, but not maternal underweight, had a significant association with CHD risk in offspring. These findings corroborated the findings in a previous meta-analysis (Zhu et al., 2018). These conclusions might be explained by the fact that maternal BMI was closely correlated with the intake of trans-fatty acids, and increased folate level could result in down-regulation of homocysteine (Davis et al., 2014). Obese pregnant women had lower folate and glutathione intake, which could lead to up-regulation of homocysteine level (Amirkhizi et al., 2014; Igosheva et al., 2010; Sanchez-Margalet et al., 2002; Vayá et al., 2012), thereby compromising the in-utero environment and impairing fetal development. Obstructive heart defect (OHD) is associated with variations in genes involved in homocysteine, folate and glutathione synthesis by the transsulfuration pathways. In addition, single nucleotide polymorphisms (SNPs) of multiple genes including genes encoding methylenetetrahydrofolate reductase, glutamate-cysteine ligase, betaine-homocysteine methyltransferase and DNA (cytosine-5-)-methyltransferase 3 beta were found to be related to increased OHD risk in females with obesity (Tang et al., 2015a). However, the association between maternal obesity and VSD risk was shown not to be statistically significant, which needed further investigation. Obese women might be susceptible to metabolic alterations, such as increased estrogen levels, hyperinsulinemia, hypertension, hyperglycemia and nutritional deficits, which increased the risk of congenital anomalies (Watkins et al., 2003). Abdominal adipose tissue accumulation was linked to the pathogenesis of diabetes, inflammation and metabolic disorders (Shaw & Carmichael, 2008). Abnormal glucose metabolism alone did not account for the elevated rates of congenital malformations in the offspring of obese women (Brite et al., 2014). It has been reported that obesity and diabetes can promote a variety of metabolic alterations, including abnormal lipid and carbohydrate metabolism, insulin resistance, altered activities of adipocyte hormones (Mills et al., 2010), disturbance in micronutrient metabolism, and elevated oxidative stress (Rankin et al., 2010). The intrauterine environment could be affected by nutritional and chemical changes during gestation. Elevated amounts of cytokines (such as interleukins, tumor necrosis factor-α, and monocyte chemotactic protein-1), leptin, procoagulant proteins and protein hormones were found in obese women, which increased the odds of maternal diseases and neonatal complications (Iessa & Bérard, 2015). In addition, obesity, insulin resistance and CHD such as myocardial contractile anomalies and cardiac hypertrophy in offspring could be caused by high fat diet exposure and maternal obesity (Dong et al., 2013).
This study showed that maternal alcohol intake was not associated with CHD risk in children. However, a potentially harmful impact of maternal alcohol intake on CHD risk was detected when pooling cohort studies. The findings of this study were consistent with those in previous meta-analysis (Sun et al., 2015). These conclusions might be explained by the fact that prenatal alcohol exposure could induce birth abnormalities, collectively referred to as fetal alcohol syndrome, and nearly 54% of live-born infants with this syndrome presented with cardiac anomalies (Karunamuni et al., 2014). In addition, maternal alcohol intake could modulate Wnt/β-catenin signaling, activating abnormal gene expressions in cardiogenesis (Serrano et al., 2010). As shown above, maternal smoking increased CHD risk in offspring, which was likely due to the teratogenic effect of smoking. Maternal cigarette exposure or even direct seminal fluid smoke exposure can cause genotoxicity (Gianicolo et al., 2010). Maternal smoking could affect the fetus due to the complex interaction of nicotine with fetal neurotransmitters (Paludetto et al., 2018). Fetal heart growth was hampered by abnormal DNA replication caused by toxins in cigarettes (Edwards & Gelb, 2016). Moreover, polymorphisms in maternal and fetal genes encoding excision repair cross-complementation group 1 (ERCC1), O-sialoglycoprotein endopeptidase, poly (ADP-ribose) polymerase 2 and ERCC5 were found to be associated with elevated risk of tobacco-associated CHD (Tang et al., 2015b). SNPs of genes encoding the glutathione-S-transferase (GST) family proteins that could alleviate oxidative stress, could also increase the risk of smoking-associated CHD and affect the expressions of GSTA4 and glutamate-cysteine ligase, the rate-limiting enzyme of glutathione synthesis contributing to DNA methylation and transsulfuration in the fetus. Additionally, SNPs in genes encoding replication factor c subunit 1 (fetal and maternal) and nitric oxide synthase 3 (fetal) involved in DNA synthesis were also shown to be linked with CHD risk (Edwards & Gelb, 2016). As demonstrated earlier, maternal diabetes showed a significant association with elevated CHD risk in children, corroborating previously reported findings (Hoang et al., 2017). This conclusion was likely due to the potential differences in the impacts of gestational and pre-gestational diabetes on CHD in offspring (Holing et al., 1998; Ray et al., 2001). Additionally, the prediabetic state in pregnant women could affect the occurrence and development of CHD in offspring, although factors related to prediabetes were not mentioned (Loeken, 2014; Lupo et al., 2012). Cardiac malformation in diabetic embryopathy deserves further investigation. However, the prevailing hypothesis is that excess glucose has a teratogenic effect on the developing heart. Glucose may indirectly exert this effect through the signaling pathways that control insulin sensitivity, which is the key regulator of embryogenesis and early embryonic development. Epigenetic changes resulting from histone acetylation and specific microRNA expressions affected by glucose or inherited genetic variations from diabetic women are additional possible causes of CHD (Øyen et al., 2016). Multifactorial processes seem to be associated with CHD risk in the offspring of obese women. Oxidative stress, Wnt signaling, nitric oxide and Notch signaling, the TGF-β pathway and the Hif1α pathway, which play critical roles in the early stages of cardiac development, have been implicated in diabetic embryopathy. Genetic analyses revealed the roles of ligands and receptors in cell signaling pathways (JAG1, NOTCH1 and NOTCH2), transcription factors (GATA4, TBX5 and NKX2.5), laterality pathway-related proteins (NODAL, LEFTY and CITED2) and structural proteins (ACTC1, MYH6, MYH7 and MYH11) in CHD occurrence and development (Basu & Garg, 2018). Moreover, the above results indicated that exposure of pregnant women to organic solvents was associated with CHD risk in offspring, while maternal coffee intake and irradiation did not show this relationship. Organic solvents, such as cleaning fluids, stain removers, paint thinners and nail polish removers, have been implicated to be associated with MTHFR 677 CC genotype. Meanwhile, transforming growth factor beta (TGF-β) receptor type 1 (TGFBR1) and TGFBR2 gene alterations are linked to patent ductus arteriosus (Nicoll, 2018). However, only a few studies have investigated the influence of these factors in pregnant women.
Maternal predisposing factors for CHD may expand the scope of CHD risk assessment in offspring, ultimately helping to reduce CHD incidence in children. Given that congenital heart defects in fetuses can lead to early miscarriage, the results of this study may provide better pregnancy management. In this meta-analysis, substantial heterogeneity was found across the studies assessing the influence of maternal age, alcohol intake, smoking, diabetes, coffee, irradiation, and exposure to organic solvents on CHD in offspring. Subgroup analysis revealed that study design, CHD type (ASD versus VSD) and confounders might be the sources of heterogeneity, as heterogeneity could be reduced when switching these subsets.
The limitations of the current study should be highlighted. First, most included studies were designed as an observational case–control trial, and uncontrolled selection and recall bias were inevitable. Second, different cutoff values and adjusted confounders were adopted by various studies, which might influence the estimate of CHD risk in offspring. Third, the included studies used various reference groups to investigate maternal factors, which might affect the pooled results. Fourth, this meta-analysis assessed reported studies, and publication bias was unavoidable. Fifth, the association analyses were based on pooled data, and various parameters in individual studies could not be comprehensively analyzed. Sixth, a small number of studies might have low statistical power to detect a difference between the CHD and non-CHD groups (for coffee and irradiation respectively). Substantial heterogeneity was detected across the studies on the influence of maternal exposure to organic solvents on child CHD. Furthermore, certain studies were conducted among infants with known chromosomal/genetic or maternal drug defects. These characteristic might affect other maternal factors and the outcome of CHD.
Conclusion
In conclusion, the current study indicated that maternal obesity, smoking, diabetes and exposure to organic solvents were significantly associated with elevated CHD risk in offspring. However, maternal age, underweight in pregnancy, alcohol and coffee intake and exposure to irradiation were not linked with offspring CHD risk. However, large prospective trials are warranted to confirm these findings.
Data Availability
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Code Availability
Not applicable.
References
Ades, A. E., Lu, G., & Higgins, J. P. (2005). The interpretation of random-effects meta-analysis in decision models. Medical Decision Making, 25(6), 646–654. https://doi.org/10.1177/0272989x05282643
Amirkhizi, F., Siassi, F., Djalali, M., & Shahraki, S. H. (2014). Impaired enzymatic antioxidant defense in erythrocytes of women with general and abdominal obesity. Obesity Research & Clinical Practice, 8(1), e26-34. https://doi.org/10.1016/j.orcp.2012.07.004
Bassili, A., Mokhtar, S. A., Dabous, N. I., Zaher, S. R., Mokhtar, M. M., & Zaki, A. (2000). Risk factors for congenital heart diseases in Alexandria, Egypt. European Journal of Epidemiology, 16(9), 805–814.
Basu, M., & Garg, V. (2018). Maternal hyperglycemia and fetal cardiac development: clinical impact and underlying mechanisms. Birth Defects Research, 110(20), 1504–1516. https://doi.org/10.1002/bdr2.1435
Bean, L. J., Allen, E. G., Tinker, S. W., Hollis, N. D., Locke, A. E., Druschel, C., Hobbs, C. A., O’Leary, L., Romitti, P. A., Royle, M. H., Torfs, C. P., Dooley, K. J., Freeman, S. B., & Sherman, S. L. (2011). Lack of maternal folic acid supplementation is associated with heart defects in Down syndrome: a report from the National Down Syndrome Project. Birth Defects Research Part a: Clinical and Molecular Teratology, 91(10), 885–893. https://doi.org/10.1002/bdra.22848
Begg, C. B., & Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50(4), 1088–1101.
Bell, R., Glinianaia, S. V., Tennant, P. W., Bilous, R. W., & Rankin, J. (2012). Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: A population-based cohort study. Diabetologia. https://doi.org/10.1007/s00125-012-2455-y
Best, K. E., & Rankin, J. (2016). Is advanced maternal age a risk factor for congenital heart disease? Birth Defects Research Part A: Clinical and Molecular Teratology, 106, 461–467
Botto, L. D., Mulinare, J., & Erickson, J. D. (2000). Occurrence of congenital heart defects in relation to maternal mulitivitamin use. American Journal of Epidemiology, 151(9), 878–884. https://doi.org/10.1093/oxfordjournals.aje.a010291
Brite, J., Laughon, S. K., Troendle, J., & Mills, J. (2014). Maternal overweight and obesity and risk of congenital heart defects in offspring. International Journal of Obesity, 38(6), 878–882. https://doi.org/10.1038/ijo.2013.244
Carmichael, S., Shaw, G., Yang, W., & Lammer, E. (2003). Maternal periconceptional alcohol consumption and risk for conotruncal heart defects. Birth Defects Research Part a: Clinical and Molecular Teratology, 67, 875–878.
Cedergren, M. I., Selbing, A. J., & Kallen, B. A. (2002). Risk factors for cardiovascular malformation—A study based on prospectively collected data. Scandinavian Journal of Work, Environment and Health, 28(1), 12–17.
Chou, H. H., Chiou, M. J., Liang, F. W., Chen, L. H., Lu, T. H., & Li, C. Y. (2016). Association of maternal chronic disease with risk of congenital heart disease in offspring. CMAJ 188, E438–E446
Cleary-Goldman, J., Malone, F. D., Vidaver, J., Ball, R. H., Nyberg, D. A., Comstock, C. H., Saade, G. R., Eddleman, K. A., Klugman, S., Dugoff, L., Timor-Tritsch, I. E., Craigo, S. D., Carr, S. R., Wolfe, H. M., Bianchi, D. W., D’Alton, M., FASTER Consortium. (2005). Impact of maternal age on obstetric outcome. Obstetrics and Gynecology, 105(5 Pt 1), 983–990. https://doi.org/10.1097/01.aog.0000158118.75532.51
Correa, A., Gilboa, S. M., Botto, L. D., Moore, C. A., Hobbs, C. A., Cleves, M. A., Riehle-Colarusso, T. J., Waller, D. K., Reece, E. A., National Birth Defects Prevention Study. (2012). Lack of periconceptional vitamins or supplements that contain folic acid and diabetes mellitus-associated birth defects. American Journal of Obstetrics and Gynecology, 206(3), 218.e211–213. https://doi.org/10.1016/j.ajog.2011.12.018
Cresci, M., Foffa, I., Ait-Ali, L., Pulignani, S., Gianicolo, E. A., Botto, N., Picano, E., & Andreassi, M. G. (2011). Maternal and paternal environmental risk factors, metabolizing GSTM1 and GSTT1 polymorphisms, and congenital heart disease. American Journal of Cardiology, 108(11), 1625–1631. https://doi.org/10.1016/j.amjcard.2011.07.022
Davis, W., van Rensburg, S. J., Cronje, F. J., Whati, L., Fisher, L. R., van der Merwe, L., Geiger, D., Hassan, M. S., Matsha, T., Erasmus, R. T., & Kotze, M. J. (2014). The fat mass and obesity-associated FTO rs9939609 polymorphism is associated with elevated homocysteine levels in patients with multiple sclerosis screened for vascular risk factors. Metabolic Brain Disease, 29(2), 409–419. https://doi.org/10.1007/s11011-014-9486-7
Deeks, J. J., Higgins, J. P. T., & Altman, D. G. (2008). Analyzing data and undertaking meta-analyses. The Cochrane Collaboration.
DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188.
Dolk, H., McCullough, N., Callaghan, S., Casey, F., Craig, B., Given, J., Loane, M., Lagan, B. M., Bunting, B., Boyle, B., & Dabir, T. (2020). Risk factors for congenital heart disease: The Baby Hearts Study, a population-based case-control study. PLoS One, 15(2), e0227908.
Dong, M., Zheng, Q., Ford, S. P., Nathanielsz, P. W., & Ren, J. (2013). Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. Journal of Molecular and Cellular Cardiology, 55, 111–116. https://doi.org/10.1016/j.yjmcc.2012.08.023
Edwards, J. J., & Gelb, B. D. (2016). Genetics of congenital heart disease. Current Opinion in Cardiology, 31(3), 235–241. https://doi.org/10.1097/hco.0000000000000274
Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. https://doi.org/10.1136/bmj.315.7109.629
Eidem, I., Stene, L. C., Henriksen, T., Hanssen, K. F., Vangen, S., Vollset, S. E., & Joner, G. (2010). Congenital anomalies in newborns of women with type 1 diabetes: Nationwide population-based study in Norway, 1999–2004. Acta Obstetricia Et Gynecologica Scandinavica, 89(11), 1403–1411. https://doi.org/10.3109/00016349.2010.518594
Erickson, J. D. (1991). Risk factors for birth defects: Data from the Atlanta Birth Defects Case-Control Study. Teratology, 43(1), 41–51. https://doi.org/10.1002/tera.1420430106
Ewing, C. K., Loffredo, C. A., & Beaty, T. H. (1997). Paternal risk factors for isolated membranous ventricular septal defects. American Journal of Medical Genetics, 71(1), 42–46.
Fazekas-Pongor, V., Fekete, M., Csáky-Szunyogh, M., Cseh, K., & Pénzes, M. (2021). Parental occupational exposure and congenital heart diseases in a Hungarian case-control study. International Archives of Occupational and Environmental Health, 94(3), 515–527.
Fixler, D. E., & Threlkeld, N. (1998). Prenatal exposures and congenital heart defects in Down syndrome infants. Teratology, 58(1), 6–12. https://doi.org/10.1002/(sici)1096-9926(199807)58:1%3c6::aid-tera4%3e3.0.co;2-0
Ghaderian, M., Emami-Moghadam, A. R., Khalilian, M. R., Riahi, K., & Ghaedi, F. (2014). Prepregnancy maternal weight and body mass index of children with and without congenital heart disease. Iranian Journal of Pediatrics, 24, 313–318.
Gianicolo, E. A., Cresci, M., Ait-Ali, L., Foffa, I., & Andreassi, M. G. (2010). Smoking and congenital heart disease: The epidemiological and biological link. Current Pharmaceutical Design, 16(23), 2572–2577. https://doi.org/10.2174/138161210792062849
Gilboa, S. M., Correa, A., Botto, L. D., Rasmussen, S. A., Waller, D. K., Hobbs, C. A., Cleves, M. A., Riehle-Colarusso, T. J., National Birth Defects Prevention Study. (2010). Association between prepregnancy body mass index and congenital heart defects. American Journal of Obstetrics and Gynecology, 202(1), 51.e51-51.e10. https://doi.org/10.1016/j.ajog.2009.08.005
Grewal, J., Carmichael, S. L., Ma, C., Lammer, E. J., & Shaw, G. M. (2008). Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Research Part a: Clinical and Molecular Teratology, 82(7), 519–526. https://doi.org/10.1002/bdra.20461
Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. https://doi.org/10.1136/bmj.327.7414.557
Hoang, T. T., Marengo, L. K., Mitchell, L. E., Canfield, M. A., & Agopian, A. J. (2017). Original findings and updated meta-analysis for the association between maternal diabetes and risk for congenital heart disease phenotypes. American Journal of Epidemiology, 186(1), 118–128. https://doi.org/10.1093/aje/kwx033
Hobbs, C. A., Cleves, M. A., Karim, M. A., Zhao, W., & MacLeod, S. L. (2010). Maternal folate-related gene environment interactions and congenital heart defects. Obstetrics and Gynecology, 116(2 Pt 1), 316–322. https://doi.org/10.1097/AOG.0b013e3181e80979
Hoffman, J. I., & Kaplan, S. (2002). The incidence of congenital heart disease. Journal of the American College of Cardiology, 39(12), 1890–1900. https://doi.org/10.1016/s0735-1097(02)01886-7
Holing, E. V., Beyer, C. S., Brown, Z. A., & Connell, F. A. (1998). Why don’t women with diabetes plan their pregnancies? Diabetes Care, 21(6), 889–895. https://doi.org/10.2337/diacare.21.6.889
Iessa, N., & Bérard, A. (2015). Update on prepregnancy maternal obesity: Birth defects and childhood outcomes. Journal of Pediatric Genetics, 4(2), 71–83. https://doi.org/10.1055/s-0035-1556739
Igosheva, N., Abramov, A. Y., Poston, L., Eckert, J. J., Fleming, T. P., Duchen, M. R., & McConnell, J. (2010). Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE, 5(4), e10074. https://doi.org/10.1371/journal.pone.0010074
Jacobsson, B., Ladfors, L., & Milsom, I. (2004). Advanced maternal age and adverse perinatal outcome. Obstetrics and Gynecology, 104(4), 727–733. https://doi.org/10.1097/01.AOG.0000140682.63746.be
Janssen, P. A., Rothman, I., & Schwartz, S. M. (1996). Congenital malformations in newborns of women with established and gestational diabetes in Washington State, 1984–91. Paediatric and Perinatal Epidemiology, 10(1), 52–63.
Källén, K. (1999). Maternal smoking and congenital heart defects. European Journal of Epidemiology, 15, 731–737.
Karatza, A. A., Giannakopoulos, I., Dassios, T. G., Belavgenis, G., Mantagos, S. P., & Varvarigou, A. A. (2011). Periconceptional tobacco smoking and isolated congenital heart defects in the neonatal period. International Journal of Cardiology, 148(3), 295–299. https://doi.org/10.1016/j.ijcard.2009.11.008
Karunamuni, G., Gu, S., Doughman, Y. Q., Peterson, L. M., Mai, K., McHale, Q., Jenkins, M. W., Linask, K. K., Rollins, A. M., & Watanabe, M. (2014). Ethanol exposure alters early cardiac function in the looping heart: A mechanism for congenital heart defects? American Journal of Physiology: Heart and Circulatory Physiology, 306(3), H414-421. https://doi.org/10.1152/ajpheart.00600.2013
Kirklin, J. W., Blackstone, E. H., Jonas, R. A., Shimazaki, Y., Kirklin, J. K., Mayer, J. E., Jr., Pacifico, A. D., & Castaneda, A. R. (1992). Morphologic and surgical determinants of outcome events after repair of tetralogy of Fallot and pulmonary stenosis. A two-institution study. Journal of Thoracic and Cardiovascular Surgery, 103(4), 706–723.
Kirklin, J. W., Colvin, E. V., McConnell, M. E., & Bargeron, L. M., Jr. (1990). Complete transposition of the great arteries: Treatment in the current era. Pediatric Clinics of North America, 37(1), 171–177. https://doi.org/10.1016/s0031-3955(16)36838-9
Kuciene, R., & Dulskiene, V. (2009). Maternal socioeconomic and lifestyle factors during pregnancy and the risk of congenital heart defects. Medicina, 45(11), 904–909.
Laopaiboon, M., Lumbiganon, P., Intarut, N., Mori, R., Ganchimeg, T., Vogel, J. P., Souza, J. P., Gülmezoglu, A. M., WHO Multicountry Survey on Maternal Newborn Health Research Network. (2014). Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG: an International Journal of Obstetrics and Gynaecology, 121(Suppl1), 49–56. https://doi.org/10.1111/1471-0528.12659
Li, X., Sundquist, J., Hamano, T., Zoller, B., & Sundquist, K. (2016). Neighbourhood deprivation, individual-level and familial-level socio-demographic factors and risk of congenital heart disease: A nationwide study from Sweden. International Journal of Behavioral Medicine, 23, 112–120
Liu, S., Joseph, K. S., Lisonkova, S., Rouleau, J., Van den Hof, M., Sauve, R., Kramer, M. S., Canadian Perinatal Surveillance System (Public Health Agency of Canada). (2013). Association between maternal chronic conditions and congenital heart defects: A population-based cohort study. Circulation, 128(6), 583–589. https://doi.org/10.1161/circulationaha.112.001054
Liu, X., Nie, Z., Chen, J., Guo, X., Ou, Y., Chen, G., Mai, J., Gong, W., Wu, Y., Gao, X., Qu, Y., Bell, E. M., Lin, S., & Zhuang, J. (2018). Does maternal environmental tobacco smoke interact with social-demographics and environmental factors on congenital heart defects? Environmental Pollution, 234, 214–222. https://doi.org/10.1016/j.envpol.2017.11.023
Loeken, M. R. (2014). Intersection of complex genetic traits affecting maternal metabolism, fetal metabolism, and neural tube defect risk: Looking for needles in multiple haystacks. Molecular Genetics and Metabolism, 111(4), 415–417. https://doi.org/10.1016/j.ymgme.2014.01.010
Loffredo, C. A., Wilson, P. D., & Ferencz, C. (2001). Maternal diabetes: An independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology, 64(2), 98–106. https://doi.org/10.1002/tera.1051
Lupo, P. J., Canfield, M. A., Chapa, C., Lu, W., Agopian, A. J., Mitchell, L. E., Shaw, G. M., Waller, D. K., Olshan, A. F., Finnell, R. H., & Zhu, H. (2012). Diabetes and obesity-related genes and the risk of neural tube defects in the national birth defects prevention study. American Journal of Epidemiology, 176(12), 1101–1109. https://doi.org/10.1093/aje/kws190
Madsen, N. L., Schwartz, S. M., Lewin, M. B., & Mueller, B. A. (2013). Prepregnancy body mass index and congenital heart defects among offspring: A population-based study. Congenital Heart Disease, 8, 131–141.
Malik, S., Cleves, M. A., Honein, M. A., Romitti, P. A., Botto, L. D., Yang, S., Hobbs, C. A., National Birth Defects Prevention Study. (2008). Maternal smoking and congenital heart defects. Pediatrics, 121(4), e810–e816. https://doi.org/10.1542/peds.2007-1519
Martínez-Frías, M. L., Bermejo, E., Rodríguez-Pinilla, E., & Frías, J. L. (2004). Risk for congenital anomalies associated with different sporadic and daily doses of alcohol consumption during pregnancy: A case-control study. Birth Defects Research Part a: Clinical and Molecular Teratology, 70(4), 194–200. https://doi.org/10.1002/bdra.20017
Martínez-Frías, M. L., Frías, J. P., Bermejo, E., Rodríguez-Pinilla, E., Prieto, L., & Frías, J. L. (2005). Pre-gestational maternal body mass index predicts an increased risk of congenital malformations in infants of mothers with gestational diabetes. Diabetic Medicine, 22(6), 775–781. https://doi.org/10.1111/j.1464-5491.2005.01492.x
Mateja, W. A., Nelson, D. B., Kroelinger, C. D., Ruzek, S., & Segal, J. (2012). The association between maternal alcohol use and smoking in early pregnancy and congenital cardiac defects. Journal of Women’s Health, 21(1), 26–34. https://doi.org/10.1089/jwh.2010.2582
McDonald, A. D., Armstrong, B. G., & Sloan, M. (1992). Cigarette, alcohol, and coffee consumption and congenital defects. American Journal of Public Health, 82(1), 91–93. https://doi.org/10.2105/ajph.82.1.91
Mills, J. L., Troendle, J., Conley, M. R., Carter, T., & Druschel, C. M. (2010). Maternal obesity and congenital heart defects: A population-based study. American Journal of Clinical Nutrition, 91(6), 1543–1549. https://doi.org/10.3945/ajcn.2009.28865
Murphy, J. G., Gersh, B. J., Mair, D. D., Fuster, V., McGoon, M. D., Ilstrup, D. M., McGoon, D. C., Kirklin, J. W., & Danielson, G. K. (1993). Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. New England Journal of Medicine, 329(9), 593–599. https://doi.org/10.1056/nejm199308263290901
Nicoll, R. (2018). Environmental contaminants and congenital heart defects: A re-evaluation of the evidence. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph15102096
Nielsen, G. L., Nørgard, B., Puho, E., Rothman, K. J., Sørensen, H. T., & Czeizel, A. E. (2005). Risk of specific congenital abnormalities in offspring of women with diabetes. Diabetic Medicine, 22(6), 693–696. https://doi.org/10.1111/j.1464-5491.2005.01477.x
Oddy, W. H., De Klerk, N. H., Miller, M., Payne, J., & Bower, C. (2009). Association of maternal pre-pregnancy weight with birth defects: Evidence from a case-control study in Western Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology, 49(1), 11–15. https://doi.org/10.1111/j.1479-828X.2008.00934.x
O’Leary, C. M., Elliott, E. J., Nassar, N., & Bower, C. (2013). Exploring the potential to use data linkage for investigating the relationship between birth defects and prenatal alcohol exposure. Birth Defects Research Part A: Clinical and Molecular Teratology, 97, 497–504.
Øyen, N., Diaz, L. J., Leirgul, E., Boyd, H. A., Priest, J., Mathiesen, E. R., Quertermous, T., Wohlfahrt, J., & Melbye, M. (2016). Prepregnancy diabetes and offspring risk of congenital heart disease: A nationwide cohort study. Circulation, 133(23), 2243–2253. https://doi.org/10.1161/circulationaha.115.017465
Pacifico, A. D., Kirklin, J. K., Colvin, E. V., McConnell, M. E., & Kirklin, J. W. (1990). Tetralogy of Fallot: Late results and reoperations. Seminars in Thoracic and Cardiovascular Surgery, 2(1), 108–116.
Paludetto, R., Capasso, L., & Raimondi, F. (2018). Infants of smoking mothers. Springer.
Peticca, P., Keely, E. J., Walker, M. C., Yang, Q., & Bottomley, J. (2009). Pregnancy outcomes in diabetes subtypes: How do they compare? A province-based study of Ontario, 2005–2006. Journal of Obstetrics and Gynaecology Canada, 31(6), 487–496. https://doi.org/10.1016/s1701-2163(16)34210-4
Rankin, J., Tennant, P. W., Stothard, K. J., Bythell, M., Summerbell, C. D., & Bell, R. (2010). Maternal body mass index and congenital anomaly risk: A cohort study. International Journal of Obesity, 34(9), 1371–1380. https://doi.org/10.1038/ijo.2010.66
Ray, J. G., O’Brien, T. E., & Chan, W. S. (2001). Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: A meta-analysis. QJM, 94(8), 435–444. https://doi.org/10.1093/qjmed/94.8.435
Salem Yaniv, S., Levy, A., Wiznitzer, A., Holcberg, G., Mazor, M., & Sheiner, E. (2011). A significant linear association exists between advanced maternal age and adverse perinatal outcome. Archives of Gynecology and Obstetrics, 283(4), 755–759. https://doi.org/10.1007/s00404-010-1459-4
Sanchez-Margalet, V., Valle, M., Ruz, F. J., Gascon, F., Mateo, J., & Goberna, R. (2002). Elevated plasma total homocysteine levels in hyperinsulinemic obese subjects. The Journal of Nutritional Biochemistry, 13(2), 75–79. https://doi.org/10.1016/s0955-2863(01)00197-8
Serrano, M., Han, M., Brinez, P., & Linask, K. K. (2010). Fetal alcohol syndrome: Cardiac birth defects in mice and prevention with folate. American Journal of Obstetrics and Gynecology, 203(1), 75.e77-75.e15. https://doi.org/10.1016/j.ajog.2010.03.017
Sharpe, P. B., Chan, A., Haan, E. A., & Hiller, J. E. (2005). Maternal diabetes and congenital anomalies in South Australia 1986–2000: A population-based cohort study. Birth Defects Research Part a: Clinical and Molecular Teratology, 73(9), 605–611. https://doi.org/10.1002/bdra.20172
Shaw, G. M., & Carmichael, S. L. (2008). Prepregnant obesity and risks of selected birth defects in offspring. Epidemiology, 19(4), 616–620. https://doi.org/10.1097/EDE.0b013e3181761fa3
Sheffield, J. S., Butler-Koster, E. L., Casey, B. M., McIntire, D. D., & Leveno, K. J. (2002). Maternal diabetes mellitus and infant malformations. Obstetrics and Gynecology, 100(5 Pt 1), 925–930. https://doi.org/10.1016/s0029-7844(02)02242-1
Smedts, H. P., de Vries, J. H., Rakhshandehroo, M., Wildhagen, M. F., Verkleij-Hagoort, A. C., Steegers, E. A., & Steegers-Theunissen, R. P. (2009). High maternal vitamin E intake by diet or supplements is associated with congenital heart defects in the offspring. BJOG: an International Journal of Obstetrics and Gynaecology, 116(3), 416–423. https://doi.org/10.1111/j.1471-0528.2008.01957.x
Smedts, H. P., van Uitert, E. M., Valkenburg, O., Laven, J. S., Eijkemans, M. J., Lindemans, J., Steegers, E. A., & Steegers-Theunissen, R. P. (2012). A derangement of the maternal lipid profile is associated with an elevated risk of congenital heart disease in the offspring. Nutrition, Metabolism, and Cardiovascular Diseases, 22(6), 477–485. https://doi.org/10.1016/j.numecd.2010.07.016
Steinberger, E. K., Ferencz, C., & Loffredo, C. A. (2002). Infants with single ventricle: A population-based epidemiological study. Teratology, 65(3), 106–115. https://doi.org/10.1002/tera.10017
Stillbirth Collaborative Research Network Writing Group. (2011). Association between stillbirth and risk factors known at pregnancy confirmation. JAMA, 306(22), 2469–2479. https://doi.org/10.1001/jama.2011.1798
Strandberg-Larsen, K., Skov-Ettrup, L. S., Grønbaek, M., Andersen, A. M., Olsen, J., & Tolstrup, J. (2011). Maternal alcohol drinking pattern during pregnancy and the risk for an offspring with an isolated congenital heart defect and in particular a ventricular septal defect or an atrial septal defect. Birth Defects Research Part a: Clinical and Molecular Teratology, 91(7), 616–622. https://doi.org/10.1002/bdra.20818
Sun, J., Chen, X., Chen, H., Ma, Z., & Zhou, J. (2015). Maternal alcohol consumption before and during pregnancy and the risks of congenital heart defects in offspring: A systematic review and meta-analysis. Congenital Heart Disease, 10(5), E216-224. https://doi.org/10.1111/chd.12271
Tang, X., Cleves, M. A., Nick, T. G., Li, M., MacLeod, S. L., Erickson, S. W., Li, J., Shaw, G. M., Mosley, B. S., Hobbs, C. A., National Birth Defects Prevention Study. (2015a). Obstructive heart defects associated with candidate genes, maternal obesity, and folic acid supplementation. American Journal of Medical Genetics Part A, 167(6), 1231–1242. https://doi.org/10.1002/ajmg.a.36867
Tang, X., Hobbs, C. A., Cleves, M. A., Erickson, S. W., MacLeod, S. L., & Malik, S. (2015b). Genetic variation affects congenital heart defect susceptibility in offspring exposed to maternal tobacco use. Birth Defects Research Part a: Clinical and Molecular Teratology, 103(10), 834–842. https://doi.org/10.1002/bdra.23370
Tikkanen, J., & Heinonen, O. P. (1991). Maternal exposure to chemical and physical factors during pregnancy and cardiovascular malformations in the offspring. Teratology, 43(6), 591–600. https://doi.org/10.1002/tera.1420430614
Tobias, A. (1999). Assessing the influence of a single study in meta-analysis. Stata Tech Bull, 47, 15–17.
Torfs, C. P., & Christianson, R. E. (1999). Maternal risk factors and major associated defects in infants with Down syndrome. Epidemiology, 10(3), 264–270.
van Beynum, I. M., Kapusta, L., Bakker, M. K., den Heijer, M., Blom, H. J., & de Walle, H. E. (2010). Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: A registry-based case-control study in the northern Netherlands. European Heart Journal, 31(4), 464–471. https://doi.org/10.1093/eurheartj/ehp479
van der Linde, D., Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A., Takkenberg, J. J., & Roos-Hesselink, J. W. (2011). Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241–2247. https://doi.org/10.1016/j.jacc.2011.08.025
van Driel, L. M., de Jonge, R., Helbing, W. A., van Zelst, B. D., Ottenkamp, J., Steegers, E. A., & Steegers-Theunissen, R. P. (2008). Maternal global methylation status and risk of congenital heart diseases. Obstetrics and Gynecology, 112(2 Pt 1), 277–283. https://doi.org/10.1097/AOG.0b013e31817dd058
Vayá, A., Rivera, L., Hernández-Mijares, A., de la Fuente, M., Solá, E., Romagnoli, M., Alis, R., & Laiz, B. (2012). Homocysteine levels in morbidly obese patients: Its association with waist circumference and insulin resistance. Clinical Hemorheology and Microcirculation, 52(1), 49–56. https://doi.org/10.3233/ch-2012-1544
Vereczkey, A., Gerencser, B., Czeizel, A. E., Szabo, I. (2014). Association of certain chronic maternal diseases with the risk of specific congenital heart defects: A population-based study. European Journal of Obstetrics & Gynecology and Reproductive Biology, 182, 1–6.
Waller, D. K., Shaw, G. M., Rasmussen, S. A., Hobbs, C. A., Canfield, M. A., Siega-Riz, A. M., Gallaway, M. S., Correa, A., National Birth Defects Prevention Study. (2007). Prepregnancy obesity as a risk factor for structural birth defects. Archives of Pediatrics and Adolescent Medicine, 161(8), 745–750. https://doi.org/10.1001/archpedi.161.8.745
Wasserman, C. R., Shaw, G. M., O’Malley, C. D., Tolarova, M. M., & Lammer, E. J. (1996). Parental cigarette smoking and risk for congenital anomalies of the heart, neural tube, or limb. Teratology, 53(4), 261–267. https://doi.org/10.1002/(sici)1096-9926(199604)53:4%3c261::aid-tera9%3e3.0.co;2-5
Watkins, Ml., & Botto, L. D. (2001). Maternal prepregnancy weight and congenital heart defects in the offspring. Epidemiology, 11(4), 439–446.
Watkins, M. L., Rasmussen, S. A., Honein, M. A., Botto, L. D., & Moore, C. A. (2003). Maternal obesity and risk for birth defects. Pediatrics, 111(5 Pt 2), 1152–1158.
Wells, G., Shea B. O., & Connell, D. (2009). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. Retrieved May 31, 2021, from https://www.ohri.ca//programs/clinical_epidemiology/oxford.htm
Williams, L. J., Correa, A., & Rasmussen, S. (2004). Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Research Part a: Clinical and Molecular Teratology, 70(2), 59–64. https://doi.org/10.1002/bdra.10145
Woods, S. E., & Raju, U. (2001). Maternal smoking and the risk of congenital birth defects: A cohort study. Journal of the American Board of Family Practice, 14(5), 330–334.
Wu, Y., Liu, B., Sun, Y., Du, Y., Santillan, M. K., Santillan, D. A., Snetselaar, L. G., Bao, W. (2020). Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care, 43(12), 2983–2990.
Zhao, M., Diao, J., Huang, P., Li, J., Li, Y., Yang, Y., Luo, L., Zhang, S., Chen, L., Wang, T., Zhu, P., Qin, J. (2020). Association of maternal diabetes mellitus and polymorphisms of the NKX2.5 gene in children with congenital heart disease: A single centre-based case-control study. Journal of Diabetes Research, 2020, 3854630.
Zhu, Y., Chen, Y., Feng, Y., Yu, D., & Mo, X. (2018). Association between maternal body mass index and congenital heart defects in infants: A meta-analysis. Congenital Heart Disease, 13(2), 271–281. https://doi.org/10.1111/chd.12567
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LW and YL carried out the studies, participated in collecting data, and drafted the manuscript. NL performed the statistical analysis and participated in its design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, L., Li, N. & Liu, Y. Association Between Maternal Factors and Risk of Congenital Heart Disease in Offspring: A Systematic Review and Meta-Analysis. Matern Child Health J 27, 29–48 (2023). https://doi.org/10.1007/s10995-022-03538-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-022-03538-8