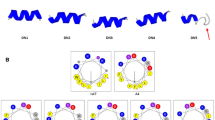
Abstract
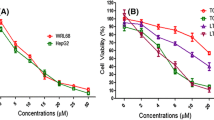
Liver cancer is a worldwide issue that also affects the Malaysian population. The occurrence is closely related to risk factors like chronic infections and environmental exposures. Due to the toxicity of conventional therapeutic drugs for liver cancer, bioactive peptides have emerged as a popular alternative anticancer agent. Although the full-length pardaxin from Pardachirus marmoratus was proven with anticancer effects, its concurrent haemolytic effects are yet to be resolved. Therefore, this study utilized in silico and in vitro analyses to assess cytotoxic effects induced by the shortened pardaxin derivatives. The in silico findings led to the discovery of a series of shortened pardaxin derivatives with 13 amino acids, where single residue replacement prediction by bioinformatics tools was done on the shortened sequences. Among the top five shortened derivatives, the derivative where amino acid threonine was replaced by proline, was identified as the most potential candidate, namely LL13. The LL13 peptide was predicted with improved anticancer effects, non-toxic, and alleviated haemolytic effects as compared to its parental peptide. The subsequent cytotoxicity testing further validated its selective toxicity against liver cancer cells, HepG2 cells, with relatively lower killing effects on the normal cells, Vero cells. These in vitro findings validated the in silico predictions and also indicated that this peptide has potential as an anticancer drug with selective targeting capabilities. In conclusion, this study has highlighted the potential of using a combination of in silico and in vitro approaches to discover potentially shortened peptides as a novel therapeutic option for liver cancer treatment.


Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- HCC:
-
Hepatocellular carcinoma
- CCA:
-
Cholangiocarcinoma
- SVM:
-
Support vector machine
- CPP:
-
Cell-penetrating peptide
- PDB:
-
Protein Data Bank
- ATCC:
-
American Type Culture Collection
- DMEM:
-
Dulbecco's Modified Eagle's Medium
- FBS:
-
Fetal bovine serum
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- DMSO:
-
Dimethyl sulfoxide
- IC50 :
-
Half maximal inhibitory concentration
- SEM:
-
Standard error of mean
- AntiCP:
-
Anticancer peptide
- FASL:
-
Fas ligand
- FAS-FADD:
-
Fas-associated protein with death domain
- ROS:
-
Reactive oxygen species
- UPR:
-
Unfolded protein response
References
Agrawal P, Bhagat D, Mahalwal M, Sharma N, Raghava GPS (2021) AntiCP 2.0: an updated model for predicting anticancer peptides. Brief Bioinform 22:3. https://doi.org/10.1093/bib/bbaa153
Ahmaditaba MA, Shahosseini S, Daraei B, Zarghi A, Houshdar Tehrani MH (2017) Design, synthesis, and biological evaluation of new peptide analogues as selective COX-2 inhibitors. Arch Pharm (weinheim) 350:10. https://doi.org/10.1002/ardp.201700158
Akagi T, Kaneko T, Kida T, Akashi M (2005) Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-phenylalanine as a protein carrier. J Control Release 108(2–3):226–236. https://doi.org/10.1016/j.jconrel.2005.08.003
Antoniou N, Vlachakis D, Memou A, Leandrou E, Valkimadi PE, Melachroinou K, Re DB, Przedborski S, Dauer WT, Stefanis L, Rideout HJ (2018) A motif within the armadillo repeat of Parkinson’s-linked LRRK2 interacts with FADD to hijack the extrinsic death pathway. Sci Rep 8(1):3455. https://doi.org/10.1038/s41598-018-21931-8
Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y (2020) Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 10(9):2993–3036
Chen YP, Shih PC, Feng CW, Wu CC, Tsui KH, Lin YH, Kuo HM, Wen ZH (2021) Pardaxin activates excessive mitophagy and mitochondria-mediated apoptosis in human ovarian cancer by inducing reactive oxygen species. Antioxidants (basel) 10:12. https://doi.org/10.3390/antiox10121883
Chiangjong W, Chutipongtanate S, Hongeng S (2020) Anticancer peptide: physicochemical property, functional aspect and trend in clinical application (Review). Int J Oncol 57(3):678–696. https://doi.org/10.3892/ijo.2020.5099
Duvvuri M, Konkar S, Hong KH, Blagg BS, Krise JP (2006) A new approach for enhancing differential selectivity of drugs to cancer cells. ACS Chem Biol 1(5):309–315. https://doi.org/10.1021/cb6001202
Fan R, Yuan Y, Zhang Q, Zhou XR, Jia L, Liu Z, Yu C, Luo SZ, Chen L (2017) Isoleucine/leucine residues at “a” and “d” positions of a heptad repeat sequence are crucial for the cytolytic activity of a short anticancer lytic peptide. Amino Acids 49(1):193–202. https://doi.org/10.1007/s00726-016-2350-9
Garcia-Oliveira P, Otero P, Pereira AG, Chamorro F, Carpena M, Echave J, Fraga-Corral M, Simal-Gandara J, Prieto MA (2021) Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals (basel) 14:2. https://doi.org/10.3390/ph14020157
Ginting TE, Suryatenggara J, Christian S, Mathew G (2017) Proinflammatory response induced by Newcastle disease virus in tumor and normal cells. Oncolytic Virother 6:21–30. https://doi.org/10.2147/OV.S123292
Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Open Source Drug Discovery C, Raghava GP (2013) In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 8(9):e73957. https://doi.org/10.1371/journal.pone.0073957
Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GP (2015) Peptide toxicity prediction. Methods Mol Biol 1268:143–157. https://doi.org/10.1007/978-1-4939-2285-7_7
Han Y, Cui Z, Li YH, Hsu WH, Lee BH (2015) In vitro and in vivo anticancer activity of pardaxin against proliferation and growth of oral squamous cell carcinoma. Mar Drugs 14(1):2. https://doi.org/10.3390/md14010002
Hsu JC, Lin LC, Tzen JT, Chen JY (2011) Pardaxin-induced apoptosis enhances antitumor activity in HeLa cells. Peptides 32(6):1110–1116. https://doi.org/10.1016/j.peptides.2011.04.024
Huang TC, Lee JF, Chen JY (2011) Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT-1080 cells. Mar Drugs 9(10):1995–2009. https://doi.org/10.3390/md9101995
Huang YP, Hsia TC, Yeh CA, Ma YS, Hsu SY, Liu YC, Lyu PC, Lai KC, Peng SF, Lien JC, Hsieh WT (2023) PW06 triggered Fas-FADD to induce apoptotic cell death in human pancreatic carcinoma MIA PaCa-2 cells through the activation of the caspase-mediated pathway. Oxid Med Cell Longev 2023:3479688. https://doi.org/10.1155/2023/3479688
Kalafatovic D, Giralt E (2017) Cell-penetrating peptides: design strategies beyond primary structure and amphipathicity. Molecules 22:11. https://doi.org/10.3390/molecules22111929
Kobon ET, Thongararm P, Roytrakul S, Meesuk L, Chumnanpuen P (2016) Prediction of anticancer peptides against MCF-7 breast cancer cells from the peptidomes of Achatina fulica mucus fractions. Comput Struct Biotechnol J 14:49–57. https://doi.org/10.1016/j.csbj.2015.11.005
Kumar V, Agrawal P, Kumar R, Bhalla S, Usmani SS, Varshney GC, Raghava GPS (2018) Prediction of cell-penetrating potential of modified peptides containing natural and chemically modified residues. Front Microbiol 9:725. https://doi.org/10.3389/fmicb.2018.00725
Lamiable A, Thevenet P, Rey J, Vavrusa M, Derreumaux P, Tuffery P (2016) PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucl Acids Res 44(W1):W449-454. https://doi.org/10.1093/nar/gkw329
Lazarovici P (2002) The structure and function of pardaxin. J Toxicol Toxin Rev 21(4):391–421. https://doi.org/10.1081/TXR-120014410
Lerksuthirat T, On-Yam P, Chitphuk S, Stitchantrakul W, Newburg DS, Morrow AL, Hongeng S, Chiangjong W, Chutipongtanate S (2023) ALA-A2 is a novel anticancer peptide inspired by alpha-lactalbumin: a discovery from a computational peptide library, in silico anticancer peptide screening and in vitro experimental validation. Glob Chall 7(3):2200213. https://doi.org/10.1002/gch2.202200213
Lin MC, Hui CF, Chen JY, Wu JL (2013) Truncated antimicrobial peptides from marine organisms retain anticancer activity and antibacterial activity against multidrug-resistant Staphylococcus aureus. Peptides 44:139–148. https://doi.org/10.1016/j.peptides.2013.04.004
Liscano Y, Onate-Garzon J, Delgado JP (2020) Peptides with dual antimicrobial-anticancer activity: strategies to overcome peptide limitations and rational design of anticancer peptides. Molecules 25:18. https://doi.org/10.3390/molecules25184245
Liu M, Lv J, Chen L, Li W, Han W (2022) In Silico discovery of anticancer peptides from Sanghuang. Int J Mol Sci 23:22. https://doi.org/10.3390/ijms232213682
Mphahlele MJ, Magwaza NM, Malindisa ST, Choong YS (2021) Biological evaluation the 2-aryl-2,3-dihydrobenzodiazaborinin-4(1H)-ones as potential dual alpha-glucosidase and alpha-amylase inhibitors with antioxidant properties. Chem Biol Drug Des 98(2):234–247. https://doi.org/10.1111/cbdd.13893
Ndolo RA, Luan Y, Duan S, Forrest ML, Krise JP (2012) Lysosomotropic properties of weakly basic anticancer agents promote cancer cell selectivity in vitro. PLoS ONE 7(11):e49366. https://doi.org/10.1371/journal.pone.0049366
Newcomb EW, Sosnow M, Demopoulos RI, Zeleniuch-Jacquotte A, Sorich J, Speyer JL (1999) Expression of the cell cycle inhibitor p27KIP1 is a new prognostic marker associated with survival in epithelial ovarian tumors. Am J Pathol 154(1):119–125. https://doi.org/10.1016/S0002-9440(10)65258-3
Ng CX, Le CF, Tor YS, Lee SH (2021) Hybrid anticancer peptides DN1 and DN4 exert selective cytotoxicity against hepatocellular carcinoma cells by inducing both intrinsic and extrinsic apoptotic pathways. Int J Pept Res Ther 27:2757–2775. https://doi.org/10.1007/s10989-021-10288-8
Niidome T, Matsuyama N, Kunihara M, Hatakeyama T, Aoyagi H (2005) Effect of chain length of cationic model peptides on antibacterial activity. Bull Chem Soc Jpn 78(3):473–476. https://doi.org/10.1246/bcsj.78.473
Oren Z, Shai Y (1996) A class of highly potent antibacterial peptides derived from pardaxin, a pore-forming peptide isolated from Moses sole fish Pardachirus marmoratus. Eur J Biochem 237(1):303–310. https://doi.org/10.1111/j.1432-1033.1996.0303n.x
Pfeffer CM, Singh ATK (2018) Apoptosis: a target for anticancer therapy. Int J Mol Sci 19:2. https://doi.org/10.3390/ijms19020448
Raihan R, Azzeri A, Mohamed R (2018) Hepatocellular carcinoma in Malaysia and its changing trend. Euroasian J Hepatogastroenterol 8(1):54–56. https://doi.org/10.5005/jp-journals-10018-1259
Raihan J, Ahmad U, Yong YK, Eshak Z, Othman F, Ideris A (2019) Regression of solid breast tumours in mice by Newcastle disease virus is associated with production of apoptosis related-cytokines. BMC Cancer 19(1):315. https://doi.org/10.1186/s12885-019-5516-5
Sayed AR, Gomha SM, Abd El-lateef HM, Abolibda TZ (2021) l-proline catalyzed green synthesis and anticancer evaluation of novel bioactive benzil bis-hydrazones under grinding technique. Green Chem Lett Rev 14(2):180–189. https://doi.org/10.1080/17518253.2021.1893392
Setiawati A (2017) Cytotoxic of Ganoderma lucidum in colon cancer through cyclooxygenase 2 (COX-2) as its molecular target. J Trop Life Sci 7:2. https://doi.org/10.11594/jtls.07.02.14
Shai Y, Fox J, Caratsch C, Shih YL, Edwards C, Lazarovici P (1988) Sequencing and synthesis of pardaxin, a polypeptide from the Red Sea Moses sole with ionophore activity. FEBS Lett 242(1):161–166. https://doi.org/10.1016/0014-5793(88)81007-x
Suarez-Jimenez GM, Burgos-Hernandez A, Ezquerra-Brauer JM (2012) Bioactive peptides and depsipeptides with anticancer potential: sources from marine animals. Mar Drugs 10(5):963–986. https://doi.org/10.3390/md10050963
Thennarasu S, Nagaraj R (1996) Specific antimicrobial and hemolytic activities of 18-residue peptides derived from the amino terminal region of the toxin pardaxin. Protein Eng 9(12):1219–1224. https://doi.org/10.1093/protein/9.12.1219
Timmons PB, Hewage CM (2020) HAPPENN is a novel tool for hemolytic activity prediction for therapeutic peptides which employs neural networks. Sci Rep 10(1):10869. https://doi.org/10.1038/s41598-020-67701-3
Timmons PB, Hewage CM (2021) ENNAACT is a novel tool which employs neural networks for anticancer activity classification for therapeutic peptides. Biomed Pharmacother 133:111051. https://doi.org/10.1016/j.biopha.2020.111051
Vishal PK, Oh JM, Khames A, Abdelgawad MA, Nair AS, Nath LR, Gambacorta N, Ciriaco F, Nicolotti O, Kim H, Mathew B (2021) Trimethoxylated halogenated chalcones as dual inhibitors of MAO-B and BACE-1 for the treatment of neurodegenerative disorders. Pharmaceutics 13:6. https://doi.org/10.3390/pharmaceutics13060850
Wang C, Dong S, Zhang L, Zhao Y, Huang L, Gong X, Wang H, Shang D (2017) Cell surface binding, uptaking and anticancer activity of L-K6, a lysine/leucine-rich peptide, on human breast cancer MCF-7 cells. Sci Rep 7(1):8293. https://doi.org/10.1038/s41598-017-08963-2
Wu SP, Huang TC, Lin CC, Hui CF, Lin CH, Chen JY (2012) Pardaxin, a fish antimicrobial peptide, exhibits antitumor activity toward murine fibrosarcoma in vitro and in vivo. Mar Drugs 10(8):1852–1872. https://doi.org/10.3390/md10081852
Zaman S, Wang R, Gandhi V (2014) Targeting the apoptosis pathway in hematologic malignancies. Leuk Lymphoma 55(9):1980–1992. https://doi.org/10.3109/10428194.2013.855307
Zhang CY, Liu S, Yang M (2022) Regulatory T cells and their associated factors in hepatocellular carcinoma development and therapy. World J Gastroenterol 28(27):3346–3358. https://doi.org/10.3748/wjg.v28.i27.3346
Zheng S, Zhu N, Shi C, Zheng H (2020) Genomic data mining approaches for the discovery of anticancer peptides from Ganoderma sinense. Phytochemistry 179:112466. https://doi.org/10.1016/j.phytochem.2020.112466
Zhou P, Jin B, Li H, Huang SY (2018) HPEPDOCK: a web server for blind peptide-protein docking based on a hierarchical algorithm. Nucl Acids Res 46(W1):W443–W450. https://doi.org/10.1093/nar/gky357
(2020) GraphPad Prism version 8.0.0 for Windows. G. Software. http://www.graphpad.com
Fayed L (2021) Caused and risk factors of liver cancer. https://www.verywellhealth.com/liver-cancer-causes-and-risk-factors-514173
Kalantari A, Farashi Bonab S, Keyvanfar H, Mortazavi P (2020) Evaluation of apoptosis induction by Newcastle disease virus LaSota strain in human breast carcinoma cells. Arch Razi Inst 75(3):367–376.https://doi.org/10.22092/ari.2019.125824.1322
Microsoft Corporation (2018) Microsoft Excel [Internet]. https://office.microsoft.com/excel
Tyagi A, Tuknait A, Anand P, Gupta S, Sharma M, Mathur D, Joshi A, Singh S, Gautam A and Raghava GP (2015) CancerPPD: a database of anticancer peptides and proteins. Nucl Acids Res 43(Database issue):D837–843. https://doi.org/10.1093/nar/gku892
Acknowledgements
This work was supported by Taylor’s University through its TAYLOR’S RESEARCH SCHOLARSHIP Programme and National Cancer Council Malaysia (MAKNA) Cancer Research Collaboration Fund 2022 (MAKNA/2022/SBS/001).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
KMW and YHW has contributed equally to this study. KMW conducted in vitro validation assays. YHW carried out the in silico predictions. KMW and YHW drafted and edited the manuscript. SHL conceived, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval and consent to participate
Not applicable.
Human and animal rights
Nor applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wong, K.M., Wong, Y.H. & Lee, S.H. In Silico Discovery of LL13, a Shortened Pardaxin 6 Peptide Derivative with Anti-proliferative Activity. Int J Pept Res Ther 30, 38 (2024). https://doi.org/10.1007/s10989-024-10615-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-024-10615-9