Abstract
Carnosine (β-Alanyl-L-Histidine) is a naturally occurring endogenous dipeptide and over-the-counter dietary supplement with a multimodal mechanism of action. The use of carnosine and its analogues in is as diverse as its mode of action and application. Carnosine’s ready availability and protective properties make it an interesting candidate for clinical use. We have now examined the mode of use in registered clinical studies. In a cross-sectional study, we evaluated the status of clinical studies on carnosine and carnosine analogues. We searched all 16 primary clinical trials registries listed in the WHO Clinical Trials Registry. Registered studies to published studies were identified and the ratio of published/unpublished studies as well as the time to publication and thematic focus were evaluated. The 16 selected registries listed 70 studies on carnosine, of which 34 have been completed and 25 have been published to date, with an average time to publication of 28 months. Carnosine/carnosine analogues were used as dietary supplements in 56% of the studies. Twelve studies were clinical trials in healthy volunteers that focused on dietary changes and underlying physiology. The other 22 studies deal with various clinical pictures, in particular metabolic and psychological disorders. This structured evaluation shows that the applications of carnosine are very versatile, and the registration in one of the clinical registries and the timely publication would facilitate the planning of further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carnosine (ß-alanyl-L-histidine), an over-the-counter food supplement, belongs to the group of naturally occurring histidine-containing dipeptides. In mammals and humans, carnosine is one of the most abundant dipeptides with the highest concentration in muscle (Boldyrev et al. 2013). In experimental settings, carnosine has been shown to exert a variety of cytoprotective functions, such as anti-glycation and antioxidant effects (Hou 2003), HNE quenching (Aldini et al. 2021), methylglyoxal polymerisation (Brings et al. 2017; Weigand et al. 2018) and contributes to intramuscular buffer capacity (de Brandt et al. 2022). Carnosine supplementation has been suggested as a possible strategy to prevent and treat various diseases in animals (Hajimoradi et al. 2023; Peters et al. 2020; Rašković et al. 2023; Spaas et al. 2021) and humans (Cesak et al. 2023; de Camargo et al. 2023; de Jager et al. 2022; Schön et al. 2023; Solana-Manrique et al. 2022; Spaas et al. 2021). The ready availability of carnosine and its protective properties make it an interesting candidate for clinical use (Caruso 2022; Feehan et al. 2022; Menon et al. 2022).
The registration of clinical trials contributes significantly to improving the transparency of clinical trials. This reduces publication bias and increases selective reporting (Aslam et al. 2013), and has been considered an ethical and scientific obligation (Venugopal and Saberwal 2021). Since a positive intervention result is as twice as likely to be published as a negative one (Song et al. 2010), we assessed the publication status of clinical trials. We identified the clinical context in which carnosine was studied and the proportion of studies in which carnosine (or carnosine analogues) was administered.
The therapeutic potential of carnosine is increasingly being investigated in in vivo studies. Therefore, we examined the clinical studies on carnosine in terms of registration, publication status/time to publication, study site and subject areas. The aim was to validate and structure the therapeutic potential of this promising molecule to provide a basis for systematic future targeted studies.
Methods
Identification and Characterization of Studies
To identify clinical trials on carnosine, on March 3, 2022 we performed a search of all 16 primary clinical trials in the WHO clinical trial registry (Juneja et al. 2019), with the keyword “carnosine”—with no other restrictions, e.g., study status, study type or study phase (Suppl. Table 1). Publications accessible after March 3, 2022 were counted as unpublished for this study. To analyze the registered trials, we screened all available data from named registries (Table 1). Studies were divided in completed and ongoing/unfinished studies, with only completed studies being considered for further analysis. In addition, studies were analyzed in terms of their clinical background and clustered in terms of their reported description in health and disease. Subgroups were formed (e.g., metabolic disorders, dietary modification) for clustering when there was a scientific or medical connection between diseases or topics of interest. Study country of origin and publication status and time-to-publication were considered along with enrolment size, study phase, sponsor, and level of masking.
Search for Publication of Completed Trials
To verify that the results of the registered and completed studies were published, the NCBI data base and Google scholar were checked for the registered study identification number, principal investigator, subject and title of the study in the clinical trial registries. Where no publication was found, the responsible investigators were contacted via email for further information. Time-to-publication was calculated from actual study completion date to the timepoint when the results were published in online or printed version (the timespan is given in months). Trial completion date is defined by ClinicalTrials.gov as “the date on which the last participant in a clinical study was examined or received an intervention/treatment to collect final data for the primary outcome measures, secondary outcome measures, and adverse events (that is, the last participant's last visit). The “estimated” study completion date is the date that the researchers think will be the study completion date”.
Statistical Analysis
The ratio of published to unpublished studies in completed clinical trials in the selected clinical study registries was determined, time-to-publication (reported as median) and number of participants per year of completion of the clinical study were calculated, thematic focus was assessed. The following continuous or categorial variables were considered: Study identification numbers, study title, study type, intervention, completion date, availability of study results, enrolment, publication date and study location and sponsors/collaborators. The variable “enrolment” displayed the size of the study (included patients) and it was used for study size analysis. The variables `publication date´ and `availability of study results´ refer to publication status and were used for inclusion of the trial in the analysis of time-to-publication as well as publication status. The variable ‘study location’ refers to the geographical localisation the study was conducted and was used for respective analysis. The variable “sponsors/collaborators” refers to the monetary sponsors and were categorized as industry or university. The purpose of the clinical studies or the intervention was analysed. Statistical calculations, were performed with Graphpad prism 9. Missing values were not imputed. Sensitivity and confounders analysis were not conducted. The geographical distribution of registered clinical trials was visualized by MapChart.net.
Results
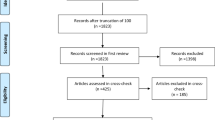
We searched all 16 WHO-listed primary clinical trial registries (Table 1) for the keyword “carnosine” and identified 70 clinical trials (Fig. 1).
Most clinical trials were registered on ClinicalTrials.gov (45) and the Iranian Clinical Trials Registry (10), followed by the Australian New Zealand Clinical Trials Registry (5), the Clinical Trials Registry – India (3) and the ISRCTN Registry (3), the German register for clinical trials (2) and the Thai register for clinical trials (2). No carnosine-related studies were found in Brazilian Clinical Trials Registry, China Clinical Trials Registry, Clinical Research Information Service, Republic of Korea, EU Clinical Trials Registry, Japan Clinical Trials Registry, Lebanese Clinical Trials Registry, Pan-African Registry for clinical trials in Peru and in Sri Lanka. At the time of analysis, 34 studies were completed, of which 24 were published on pubmed.gov (22/24) and/or Google Scholar (24/24), resulting in a publication rate of 71%. The median time to publication was 24 months and varied between 5 and 104 months (Fig. 2A) Studies published before the actual completion date are not included in the figure (NCT03087721, NCT02011100). The final year was from 2007 to 2022, most studies were interventional (97%; 33 of 34) and randomized (76%, 26 of 34) (Suppl. Table 1). Carnosine or a corresponding carnosine analogue or precursor was supplemented in 19 studies (56%; Suppl. Table 2). Median time to publication of studies with carnosine/carnosine analogue supplementation is lower (19 months) than those without supplementation (27 months). The cohort size ranged from 3 to 516 patients, the median number of enrollments per year after graduation was 43 in the years 2007–2022 (Fig. 2B). Of the completed studies, 28 were funded by academia, 5 by industry, and 1 with no listed sponsor (Fig. 2C).
A The median time to publication was 24 months and varied between 5 and 104 months. Dashed line denotes timeline mandate by the federal drug administration amendment act (FDAAA) of 2007 demanding a timespan of maximum 12 months from completion date of clinical trials to publish the data. B The number of enrollment for each study. C Number of studies funded by industry and academics
Clinical studies on carnosine exist for various diseases (22) and in healthy subjects (12) (Fig. 3). In the disease areas, the main areas of interest were metabolic disorders (7 studies), mental disorders (4 studies), cancer (3 studies) and multiple sclerosis (3 studies). The studies on carnosine metabolism in health examined effects on dietary modification (5 studies), physiology (5 studies), aging (1 study) and biomarkers (1 study).
Most registered clinical trials were conducted in the US (n = 9), followed by Belgium and Italy (n = 5). A total of 11 studies were conducted or sponsored in North and South America, compared to 19 in Europe and 4 in Africa/Asia (Fig. 4).
Discussion
Registering clinical trial offers benefits in terms of transparency, comparability, reducing publication bias, and selective reporting (Aslam et al 2013; Venugopal and Saberwal 2021). In a cross-sectional study, we evaluated the proportion of registered studies on carnosine and their publication status.
About one third of all studies focused on aging, its role as a putative biomarker or the alterations of carnosine metabolism through dietary intervention. Therapeutic use has been studied in metabolic diseases/diabetes, cancer or brain/mental disorders and nutritional interventions on muscle exercise performance have been studied in healthy people. The publication rate for the registered studies is 71%, and thus higher than previously reported (Anderson et al. 2015) where only 38% of all completed or early terminated trials registered at ClinicalTrials.gov were published. Studies on paediatric emergent delirium (45%) (Meyburg and Ries 2020), paediatric appendicitis (63%) (Breil et al. 2018) or diabetic nephropathy (63%) (Modafferi et al. 2019) found a somewhat higher publication status. The median time to publication of results was 24 months and more than 8 months beyond the time recommended by Federal Drug Administration Amendments Act of 2007 (FDAAA). With only 5 clinical trials with at least 100 participants, the enrolment number was lower compared to many other trials, such as cancer (about 300 participants (Tran et al. 2020) or paediatric appendicitis (around 230 participants) (Breil et al. 2018).
There is an urgent need to perform pharmacokinetic studies to evaluate bioavailability considering the specific routes of administration, dosages and duration of treatment. Caruso's results (Caruso 2022) and the current study will help plan future studies of the promising dipeptide carnosine. Finally, there is also a need for coordinated preclinical studies that lay the foundation for clearly defined future clinical studies. In view of the published results, the number of registered studies appears to be very small. This makes it much more difficult to make a statement about the current status. Clinical registries can only be as good as the people who use them.
Strength and Limitations
No statements of quality or outcome of the registered and in this cross-sectional study named clinical trial were performed. The heterogeneity of the studies, the different doses and durations of carnosine supplementation, the lack of gold-standard methodology to measure outcomes and the general low number of participants makes a comparison or statements about the potential of carnosine of these studies impossible.
Conclusion
This study shows the broad variety of application areas and studies with the dipeptide “carnosine”. Even though this study represents the clinical trials registered in clinical databases, the broad spectrum of study interests will be read from this study and projected to the non-registered ones. It is certainly desirable that all studies are registered so that further targeted studies and their evaluations can be carried out.
Data Availability
All data are in the manuscript.
References
Aldini G, de Courten B, Regazzoni L, Gilardoni E, Ferrario G, Baron G, Altomare A, D’Amato A, Vistoli G, Carini M (2021) Understanding the antioxidant and carbonyl sequestering activity of carnosine: direct and indirect mechanisms. Free Radic Res 55:321–330
Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM (2015) Compliance with results reporting at ClinicalTrials.gov. N Engl J Med 372:1031–1039
Aslam A, Imanullah S, Asim M, El-Menyar A (2013) Registration of clinical trials: is it really needed? N Am J Med Sci 5:713–715
Boldyrev AA, Aldini G, Derave W (2013) Physiology and pathophysiology of carnosine. Physiol Rev 93:1803–1845
Breil T, Boettcher M, Hoffmann GF, Ries M (2018) Publication status of completed registered studies in paediatric appendicitis: a cross-sectional analysis. BMJ Open 8:e021684. https://doi.org/10.1136/bmjopen-2018-021684
Brings S, Fleming T, De Buhr S, Beijer B, Lindner T, Wischnjow A, Kender Z, Peters V, Kopf S, Haberkorn U et al (2017) A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim Biophys Acta 1863:654–662
Caruso G (2022) Unveiling the hidden therapeutic potential of carnosine, a molecule with a multimodal mechanism of action: a position paper. Molecules 27(10):3303. https://doi.org/10.3390/molecules27103303
Cesak O, Vostalova J, Vidlar A, Bastlova P, Student V Jr (2023) Carnosine and beta-alanine supplementation in human medicine: narrative review and critical assessment. Nutrients 15(7):1770. https://doi.org/10.3390/nu15071770
De Brandt J, Derave W, Vandenabeele F, Pomiès P, Blancquaert L, Keytsman C, Barusso-Grüninger MS, de Lima FF, Hayot M, Spruit MA, Burtin C (2022) Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled trial. J Cachexia Sarcopenia Muscle 13(5):2361–2372
de Camargo JBB, Brigatto FA, Zaroni RS, Germano MD, Souza D, Bacurau RF, Marchetti PH, Braz TV, Aoki MS, Lopes CR (2023) Does beta-alanine supplementation enhance adaptations to resistance training? A randomized, placebo-controlled, double-blind study. Biol Sport 40:217–224
de Jager S, Blancquaert L, Van der Stede T, Lievens E, De Baere S, Croubels S, Gilardoni E, Regazzoni LG, Aldini G, Bourgois JG et al (2022) The ergogenic effect of acute carnosine and anserine supplementation: dosing, timing, and underlying mechanism. J Int Soc Sports Nutr 19:70–91
Feehan J, Hariharan R, Buckenham T, Handley C, Bhatnagar A, Baba SP, de Courten B (2022) Carnosine as a potential therapeutic for the management of peripheral vascular disease. Nutr Metab Cardiovasc Dis 32:2289–2296
Hajimoradi S, Hassanpour S, Vazir B (2023) Maternal supplementation of L-carnosine improves reflexive motor behaviors in mice offspring. Neurosci Lett 807:137266
Hou W, Chen HJ, Lin YH (2003) Antioxidant peptides with angiotensin converting enzyme inhibitory activities and applications for angiotensin converting enzyme purification. J Agric Food Chem 51:1706–17093
Juneja A, Gupta J, Yadav N, Sharma S, Panchal Y, Adhikari T, Rao MVV (2019) An overview of primary registries of WHO’s international clinical trial registry platform. Ayu 40:141–146
Menon K, de Courten B, Magliano DJ, Ademi Z, Liew D, Zomer E (2022) The cost-effectiveness of supplemental carnosine in type 2 diabetes. Nutrients 14:215. https://doi.org/10.3390/nu14010215
Meyburg J, Ries M (2020) Publication bias in pediatric emergence delirium: a cross-sectional analysis of ClinicalTrials.gov and ClinicalTrialsRegister.eu. BMJ Open 10:e037346
Modafferi S, Ries M, Calabrese V, Schmitt CP, Nawroth P, Kopf S, Peters V (2019) Clinical trials on diabetic nephropathy: a cross-sectional analysis. Diabetes Ther 10:229–243
Peters V, Yard B, Schmitt CP (2020) Carnosine and diabetic nephropathy. Curr Med Chem 27:1801–1812. https://doi.org/10.2174/0929867326666190326111851
Rašković A, Martić N, Zaklan D, Duborija-Kovačević N, Vujčić M, Andrejić-Višnjić B, Čapo I, Mijović R, Krga M, Pavlović N et al (2023) Antihyperlipidemic potential of dietary supplementation with carnosine in high-fat diet-fed rats. Eur Rev Med Pharmacol Sci 27:1083–1094
Schön M, Just I, Krumpolec P, Blažíček P, Valkovič L, Aldini G, Tsai CL, De Courten B, Krššák M, Ukropcová B et al (2023) Supplementation-induced change in muscle carnosine is paralleled by changes in muscle metabolism, protein glycation and reactive carbonyl species sequestering. Physiol Res 72:87–97
Solana-Manrique C, Sanz FJ, Martínez-Carrión G, Paricio N (2022) Antioxidant and neuroprotective effects of carnosine: therapeutic implications in neurodegenerative diseases. Antioxidants 11:848. https://doi.org/10.3390/antiox11050848
Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, Hing C, Kwok CS, Pang C, Harvey I (2010) Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess (Winchester, England) 14:1–193
Spaas J, Van Noten P, Keytsman C, Nieste I, Blancquaert L, Derave W, Eijnde BO (2021) Carnosine and skeletal muscle dysfunction in a rodent multiple sclerosis model. Amino Acids 53:1749–1761
Tran G, Harker M, Chiswell K, Unger JM, Fleury ME, Hirsch B, Miller K, d’Almada P, Tibbs S, Zafar SY (2020) Feasibility of cancer clinical trial enrollment goals based on cancer incidence. JCO Clin Cancer Inform 4:35–49
Venugopal N, Saberwal G (2021) A comparative analysis of important public clinical trial registries, and a proposal for an interim ideal one. PLoS ONE 16:e0251191
Weigand T, Singler B, Fleming T, Nawroth P, Klika KD, Thiel C, Baelde H, Garbade SF, Wagner AH, Hecker M et al (2018) Carnosine catalyzes the formation of the oligo/polymeric products of methylglyoxal. Cell Physiol Biochem 46:713–726
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–project number 236360313–SFB 1118.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception or design of the work, or the acquisition, analysis, or interpretation of data for the work: TP, KS, DW, VP, MR; Data analysis and visualization: TP, DW, KS, Writing: TP, KS, VP, MR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
None.
Ethical Approval
No human material was used in this study.
Patient Consent
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pfeffer, T., Wen, D., Stefanidis, K. et al. A Cross-Sectional Analysis of Registered Studies on the Promising Dipeptide Carnosine. Int J Pept Res Ther 29, 81 (2023). https://doi.org/10.1007/s10989-023-10553-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-023-10553-y