Abstract
Scorpion venom contains various peptides that could be utilized to treat various diseases, including cancer. This study aimed to evaluate the anti-cancer activity of scorpion venom peptide (Smp24) using a solid Ehrlich Carcinoma (SEC) mice model. SEC model was established by subcutaneous transplantation of SEC cells into Swiss albino female mice afterward subcutaneous injection of the Smp24 peptide compared to 5-Fluorouracil (5-FU) as a standard drug. Various biochemical, hematological, histopathological, immunohistochemical, and molecular (western blotting and RT-PCR) assays were performed to evaluate the antitumor activity of Smp24. Results revealed that Smp24 peptide significantly reduced tumor volume. Interestingly, Smp24 peptide significantly restored normal body functions in cancer-treated groups by maintaining HB, RBC’s, and WBC’s levels, reducing the elevated serum ALT and AST, and increasing total protein and albumin as well as enhancing antioxidant status through reducing the level of MDA and NO and elevating GSH, SOD, and CAT levels. Moreover, it restored the normal morphology of the liver and kidney tissues and improved hematological parameters in cancer-treated animals. Smp24 induced apoptosis in SEC cells, through upregulation of caspase-3 and BAX and the downregulation of VEGF, Bcl-2, p53, PCNA, and Ki67. Moreover, results exhibited the apoptotic and antiangiogenic effects of Smp24 against SEC cancer cells. These findings supported our previous results about the anti-cancer efficacy of Smp24 and made it a good candidate for developing effective and safe anti-cancer agents.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Cancer is a disease characterized by the abnormal, uncontrolled proliferation of cells, which can have both a genetic and an environmental cause (Sung et al. 2021). While many therapies have been implemented in an effort to slow the growth of cancer cells, some of these malignancies are becoming resistant to treatment as a result of new mutations. Additionally, some application procedures have unfavorable side effects, such as radiation therapy, chemotherapy, and surgery. Research efforts have accumulated to show that peptides and toxins derived from neglected natural sources, such as scorpion venoms, are being used to treat cancer. Consequently, further research studies are urgently needed to identify biologically active peptides from natural sources and develop them into effective therapeutic agents. These peptides can specifically target cancer cells and induce substantial damage to them while having little effect on healthy cells due to their structure-specificity and structural-related cytotoxicity (Uzair et al. 2018).
Venoms include a wealth of pharmacologically active compounds that are used to treat a wide range of illnesses, including cancer (Abdel-Rahman et al. 2015; Harrison et al. 2016; Ghosh et al. 2009). Several venom peptides/proteins had notable cytotoxic, antiproliferative, immunosuppressive, and apoptogenic effects on several cancer cell lines (Ejaz et al. 2018; Sarfo-Poku et al. 2016; Ortiz et al. 2015). In previous studies, scorpion venom peptides have been demonstrated to block or modify the ion channels of cancer cells. These ion channels are overexpressed or downregulated, and they play a key role in cancer growth and invasion, in addition to depolarizing immune cells. Scorpion venom also has other anti-cancer characteristics, such as inducing apoptosis and inhibiting cancer cell metastasis, invasion, and proliferation, as well as angiogenesis. In addition, there is significant evidence that scorpion venoms have immunomodulatory properties. (Mikaelian et al. 2020)
The discovery of medicines that develop the efficient destruction of cancer cells by apoptosis has been a hallmark and objective of cancer biology for the past 30 years. Numerous signaling pathways (intrinsic and extrinsic) are involved in the programmed cell death process, which are triggered by a variety of conditions such as cellular stress, DNA damage, and immune monitoring. Cell death can be influenced by the interplay of apoptosis pathways with other signaling processes. (Carneiro and El-Deiry 2020) Scorpion venom peptides have a pleiotropic effect on cancer cells by (a) inhibiting their proliferative characteristics by primarily inducing apoptosis without affecting the proliferative capabilities of healthy cells like lymphocytes, (b) interrupting cancer cell metastatic and invasion abilities, (c) suppressing angiogenesis and (d) modulation of immune cell function by reducing the inflammatory cascade. As a result of these promising findings, scorpion venom peptides may be used to target a variety of hallmarks involved in cancer cell development and invasion. (Mikaelian et al. 2020) Wong et al. illustrated the efficacy of peptides and toxins isolated from various natural sources in killing cancer cells, the selectivity of these agents towards individual anti-cancer processes, and the potential of conjugated peptides as a new therapeutic approach (Wong et al. 2015).
In vitro and in vivo, as well as in their clinical manifestations, scorpion toxins represent one of the most promising approaches for fighting cancer. Some bioactive peptides have been shown to have anti-cancer effects; they include cationic alpha helical peptides. These peptides are typically under 50 amino acids in length, and they have been shown to assume a hydrophobic nature and -helical secondary structure when they meet membranes. This allows them to adopt an amphiphilic conformation. Additionally, they also have a unique structural and morphological feature against the cancer cells (Almaaytah et al. 2013).
The cationic antimicrobial peptide known as Smp24, which was found in the venom gland of the Egyptian scorpion Scorpio maurus palmatus, has varied promising cytotoxicity on several tumors (KG1a, CCRF-CEM, and HepG2) and non-tumor (CD34+, HRECs, HACAT) cell lines (Elrayess et al. 2020). Ruiyin Guo et al. assessed the impact of Smp24 on survival, membrane disruption, cytoskeleton, migration and invasion, and MMP-2/-9 and TIMP-1/-2 expression of human lung cancer cells. Acute toxicity and in vivo anti-cancer function were also evaluated. Smp24 was discovered to exhibit low toxicity to normal cells (MRC-5) while suppressing the growth of A549, H3122, PC-9, and H460 with an IC50 value of 14.68 M. Additionally, by compromising the integrity of the cell membrane as well as the mitochondrial and nuclear membranes (Guo et al. 2022).
Nguyen et al. confirmed anti-liver cancer utilizing xenograft mice demonstrated that Smp24 penetrated HepG2 cells via hole creation and endocytosis, leading to mitochondrial dysfunctions and membrane abnormalities, which in turn caused cell necrosis, cycle arrest, apoptosis, and autophagy (Nguyen et al. 2022). Hence, in continuation of previous studies, the present study was carried out to assess the anti-cancer activity of Smp24 peptide against solid Ehrlich carcinoma-bearing mice by investigating the apoptotic and antiangiogenic activities as the cell death mechanism in a selective way.
Material and Methods
The sequence of scorpion venom peptide Smp24 (IWSFLIKAATKLLPSLFGGGKKDS) (Fig. 1) was synthesized using solid-phase chemistry, powder with purity > 95% (GL Biochem, Shanghai Ltd., China) (Abdel-Rahman et al. 2013).
Implantation of Ehrlich Carcinoma Cells and Animal Groups
Ehrlich carcinoma cells (EAC) were provided by the National Cancer Institute (Cairo University, Egypt). Serial subcutaneous transplantation of 1 × 106 viable cells in 0.3 mL physiological saline for each mouse was used to proliferate the SEC tumor in 20 days (Nafie et al. 2021, 2022).
Fifty Swiss Webster female albino mice (20–27 g, 6 weeks) were kept in plastic cages (room temperature 22 °C) with unrestricted access to regular food and water. All techniques relevant to the treatment and handling of the animals were revised and approved firstly by the Faculty of Science, Suez Canal University, research ethics committee (REC-10-2020). The experimental animals were randomly divided into five groups (n = 10, in each group). Group 1 was subcutaneously injected with physiological saline (0.1 mL/mice) and considered a negative control; Groups 2, 3 and 4 were subcutaneously injected with 1 × 106 Ehrlich carcinoma cells/mouse and served as the SEC control, Smp24-treatd, and 5-FU-treated, respectively. Group 3 (SEC + Smp24) received repetitive subcutaneous (SQ) injections of Smp24 (2 mg/Kg BW, SQ); every 48 h for two weeks at the site of the solid tumor (Nguyen et al. 2022). Group 4 (SEC + 5-FU) was administered the standard anti-cancer drug 5-fluorouracil (5-FU) (2 mg/Kg BW, SQ) (Adam et al. 2022) as a reference control. Group 5, considered a physiological group, received only the scorpion venom peptide Smp24 (2 mg/Kg BW, SQ). The body weight and survival rate of the treated and control groups were measured daily for three weeks. At the end of experiment, blood was collected from mice by cardiac puncture under mild anesthesia for hematological and biochemical assays. Finally, anesthetized mice were sacrificed, and tissues of the liver, kidney, and solid tumor were collected for measuring oxidant and antioxidant parameters and histological as well as immunohistochemical investigations.
Hematological and Biochemical Assays
The Abbott CELL-DYN® 1800 automated hematology analyzer (USA) was used to perform the complete blood count (CBC) using recommended commercial kits (Abbott Laboratories, Abbott Park, IL, USA). (Kendall et al. 2003) Levels of serum alanine aminotransferase (ALT) (Dufour et al. 2000), aspartate aminotransferase (AST) (Hafkenscheid and Dijt 1979), total protein (Doumas et al. 1981) and albumin (Hill 1985) were measured using standard commercial kits (Instrumentation Laboratory SpA, Inova diagnostics, Milano, Italy). Oxidant and antioxidant parameters were measured in phosphate-buffered liver tissue homogenate (phosphate-buffered saline solution, pH 7.4. comprising 0.16 mg /mL heparin, 50 mM potassium phosphate, 1 mM EDTA per gram tissue). The level of reduced glutathione (GSH) (Beutler 1963) and the activity of both catalase (CAT) (Aebi 1984) and superoxide dismutase (SOD) (Nishikimi et al. 1972) were calculated using standard commercial kits (Bio-diagnostic, Cairo, Egypt), as well as the contents of both MDA (Ohkawa et al. 1979) and NO (Montgomery and Dymock 1961).
Histopathological Assay
Parts of the liver and kidney were fixed in phosphate-buffered formalin at a concentration of 10%. After dehydration in ethanol and xylene, the samples were embedded in paraffin, cut into 5-m sections, and stained with Hematoxylin and Eosin for microscopic histopathological investigation. (Bancroft 2008)
Immunohistochemical Analysis of Solid Tumors
For immunohistochemical analysis, Ehrlich solid tumors were extracted, fixed in formalin, and paraffin-embedded. (Jakob et al. 2008) Endogenous peroxidases were blocked for 10 min with 3% hydrogen peroxide in PBS after antigen retrieval with 10 mM sodium citrate buffer (pH 6.0 at 80 °C for 10 min). The slides were then incubated overnight at 4 °C in a humidified chamber with primary antibodies against Ki67, caspase-3, PCNA, and VEGF, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies for 30 min at 37 °C at 1:100 dilutions and visualized with 3,3′-diaminobenzidine tetra hydrochloride reagent (Broad spectrum LAB-SA Detection System from Invitrogen Cat. No. 95-9943-B). The sections were counterstained with hematoxylin and digitally imaged. Cells with positive nuclear reaction to anti-Ki67 and PCNA were considered positive, while caspase-3 and VEGF positivity were examined in tumor cells cytoplasm. The modified Allred scoring system was used to undertake a semi-quantitative analysis of stained tissue slices. Positive cells were counted in three high-power fields (400×, with the average number calculated. To get the final grades, the percentage of positive cells (0–5 and the staining intensity of the cytoplasm (0–3 were added together. The percentage of cells that were positive was set as follows: 1-less than ten positive cells; 2-between ten and twenty positive cells; 3-between twenty and fifty positive cells; 4-between fifty and seventy positive cells; and 5-over seventy positive cells. Positive staining intensity in the cytoplasm was graded as follows: 1-weak, 2-medium, and 3-strong. (Ilić et al. 2019)
Real Time Polymerase Chain Reaction (RT-PCR)
The qRT-PCR was used to examine the expression of various genes in the tissues of solid tumors treated with Smp24: caspase-3, VEGF, Bax, Bcl-2, and β-actin (housekeeping gene) (Table 1). The total RNA was extracted from tumor samples using total RNA isolation system (Thermo Scientific, USA). High-capacity cDNA reverse transcription kit (#K4374966, Thermo Fisher Scientific, USA) was used for cDNA synthesis and SYBR Green I for the real-time qPCR with Applied Biosystem Software Version 3.1 (StepOne™, USA). All reactions were performed for 35 cycles using the following temperature profiles: “95 °C for 5 min (initial denaturation); 95 °C for 15 min (Denaturation), 55 °C for 30 min (Annealing), and 72 °C for 30 min (Extension)” (Nafie et al. 2020). All samples were normalized to ß-actin and then scaled relative to controls using the standard ΔCt method. Data was represented as fold change relative to controls. (Boraei et al. 2021; Teleb et al. 2022)
Western Blotting
Protein was extracted from tumor tissues via homogenization in radioimmunoprecipitation assay (RIPA) buffer (Bio BASIC INC, Marhham Ontario, Canada, #PL005) with freshly added proteases and phosphatases inhibitors. Protein concentration was determined using Bradford Protein Assay Kit (Bio BASIC INC, # SK3041). Total protein (5–20 µg) was separated on TGX Stain-Free™ FastCast™ Acrylamide Kit (SDS-PAGE) (Bio-Rad Laboratories, TNC, USA, #161-0181) and transferred to PVDF membranes using a Trans-Blot Turbo transfer system (BioRad). Membranes were blocked at room temperature for 1 h with gentle shaking in tris-buffered saline with tween 20 (TEST; 20 mM tris (pH7.5), 150 mM NaCl, 0.1% tween 20) containing 3% bovine serum albumin. The membranes were then probed with primary antibodies against caspase 3 (Thermo Scientific, Rockford, IL, USA, # 700182), VEGF (Thermo Scientific, # MA5-13182), PCNA (Thermo Scientific, 13-3900) and Ki-67 (Thermo Scientific, #MA5-14520) overnight at 4ºC. After washing, membranes were incubated with HRP-conjugated secondary antibodies for 1 h and developed using a chemiluminescent substrate (ClarityTM Western ECL substrate; BIO-RAD, USA, #170-5060). The chemiluminescent signals were captured using Chemi Doc MP imager (BioRad) and quantified using Image analysis software after normalization to ß-actin and reported as fold change relative to controls. (Harlow and Lane 1999)
Statistical Analysis
Data were statistically analyzed using unpaired student t-test or one-way ANOVA (GraphPad prism software version 8 for Windows). Data were expressed as Mean ± standard errors, and results were considered statistically significant when p ≤ 0.05.
Results
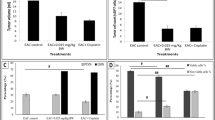
Smp24 Treatment Inhibited Tumor Volume Growth in EAC-Bearing Mice
Treatment with Smp24 peptide (2 mg/Kg BW, SQ) reduced tumor volume in SEC-bearing mice from 4552.1 to 3696.8 mm3 and induced 21.3% tumor inhibition ratio compared to 5-Fluorouracil which induced a 20.8% tumor inhibition ratio as shown in (Fig. 2). In one mouse of the treated group, the tumor approximately diminished as its volume became 62.5 mm3. These findings indicated that the Smp24 peptide has antitumor effects by controlling tumor proliferation.
Smp24 Peptide Treatment Ameliorated Hematological Parameters in EAC-Bearing Mice
As shown in (Table 2), the effects of Smp24 peptide and 5-Fluorouracil treatments on the hematological parameters of SEC-bearing mice. In SEC-bearing mice, it was found that hemoglobin content and RBC’s count were significantly decreased from 14.92 (g/dL) to 10.42 (g/dL) and from 9.09 (× 106/µL) to 6.04 (× 106/µL) respectively while WBC’s count was significantly increased from 11.12 (× 103/µL) to 20.5 (× 103/µL) compared to normal mice. Upon treatment of cancer group, it was found that hemoglobin content and RBC’s count were increased to be 13.66 (g/dl) and 9.27 (× 106/µL), respectively, while WBC’s count was decreased to be 14.84 (× 103/µL) in a similar way with 5-Fluorouracil treatment, that retained the CBC values near Smp24 treatment. Additionally, upon treatment of normal mice with the Smp24 peptide, it was found that the peptide treatment didn’t significantly change hemoglobin content and RBC’s count, and this indicated the safety of Smp24 in normal mice.
Smp24 Peptide Treatment Improved Biochemical, Oxidant and Antioxidant Parameters in EAC-Bearing Mice
For liver enzymes, in SEC-bearing mice, ALT and AST were increased from 43.48 (U/L) to 63.2 (U/L) and from 39.68 (U/L) to 59.92 (U/L), respectively, and albumin level was decreased from 3.29 (mg/dl) to 3.26 (mg/dl). Upon treatment with Smp24 peptide, the ALT and AST were decreased to 46.28 (U/L) and 44.9 (U/L), respectively, while albumin and total protein were increased to 3.35 (mg/dL) and 6.622 (mg/dL), respectively in comparison with 5-Fluorouracil treatment as shown in (Fig. 3). Smp24 treatment ameliorated liver functions with values near like 5-FU treatment, and this indicated its potential protective activity against cancer.
Effect of Smp24 peptide treatment (2 mg/Kg BW, SQ) on A) liver enzymes (U/L) B) liver proteins (mg/dL) of SEC-bearing mice. Results are expressed as mean ± SEM (n = 5). *Significant difference between the normal control and SEC control using unpaired t-test. #Significant difference between SEC control and treated groups using one-way ANOVA (P ≤ 0.05)
For liver oxidant and antioxidant parameters, the treated group with the peptide shows a significant decrease in the oxidant parameters (MDA and NO) from 130.07 to 58.4 (nmol/g. tissue) and from 89 to 21.2 (µmol/L), respectively, and a remarkable increase in the antioxidant parameters (GSH, CAT, and SOD) from 57.08 to 105.95 (mg/g. tissue) and from 35.43 to 87.63 (U/g. tissue) and from 11.01 to 28.54 (U/g. tissue), respectively as shown in (Fig. 4).
Smp24 Peptide Treatment Retained Histopathological Examination
Liver Tissues
As seen in (Fig. 5A), no histopathological alteration was observed in liver tissue of normal control group; the architecture of the hepatic lobule and the central vein surrounded by hepatocytes were recorded (Fig. 5A). The histopathological findings detected in the livers of the SEC control group were hydropic degeneration of the hepatocytes, loss of cell boundaries, and dilatation in the sinusoids (Fig. 5B). Some other hepatocytes showed nuclear pyknosis and karyolysis, as shown by the red arrows. SEC mice treated with the Smp24 peptide group showed that the hepatic lobules appeared normal. Few hepatocytes showed hydropic degeneration (Fig. 5C). SEC mice treated with 5-FU group still showed hydropic degeneration of the hepatocytes and nuclear pyknosis and karyolysis, as shown by the red arrows (Fig. 5D). The normal group, on the other hand, that was given a dose of Smp24 peptide showed normal liver and intact parenchyma as that in the normal control groups (Fig. 5E).
Kidney Tissues
Kidney tissues of normal control mice (Fig. 6A) exhibited normal histological structure with intact renal corpuscle of glomerulus and bowman's capsule and urinary space surrounded with proximal and distal convoluted tubules, as shown by the asterisk and red arrows. SEC control group (Fig. 6B) showed inflammatory infiltration in the interstitial spaces and renal corpuscles with congestion and hypercellularity associated with hyperemia in glomerular and degeneration in the lining of the epithelium of the renal tubules as shown by the asterisk and red arrows. In the SEC group that received Smp24 peptide (Fig. 6C), the renal tubules exhibit almost normal histological structure with intact renal corpuscles and tubules as in the normal control group. 5-FU-treated group (Fig. 6D) still showed mild inflammatory infiltration in the interstitial spaces and hyperemia in the intratubular intervals, as shown by the red arrow. The normal group injected with Smp24 peptide (Fig. 6E) showed normal renal corpuscle, tubules, and bowman's capsule.
Smp24 Peptide as Apoptotic and Antiangiogenic Anti-cancer agent
Immunohistochemical Examination of Caspase 3, Ki67, PCNA, and VEGF Proteins
The immunohistochemistry staining for Caspase 3, Ki67, PCNA, and VEGF is shown in (Fig. 7). Few tumor cells showed weak cytoplasmic positivity for Caspase 3 in the SEC + Smp24 group, as shown by the red arrows (Score 1), and weak nuclear staining for Ki67, as shown by the red arrows (score 1) and PCNA, as shown by the red arrows (score 3) when compared to the SEC control group (score 3, score 4, score 3, respectively) (Ishak et al. 1995). However, many tumor cells showed moderate cytoplasmic expression for VEGF, as shown by the red arrows (score 2) in SEC + Smp24 compared to the SEC control group (score 4).
As illustrated in (Table 3), the immunohistochemistry scoring for the SEC control group and the SEC + Smp24 group. The number of positive cells was significantly decreased in Ki67 from 53.3 to 5.3, in VEGF from 60.3 to 18.3, and caspase-3 from 19.67 to 1.6 in the SEC + Smp24 group in comparison to the SEC control. However, the number of positive cells in PCNA decreased from 38 to 29 in the SEC + Smp24 group compared to the SEC control group.
Gene expression using RT-PCR of Caspase3, VEGF, Bcl-2, and BAX
RT-PCR assay was done to investigate the consequence of Smp24 peptide on apoptosis. (Fig. 8) shows the effect of Smp24 peptide treatment on the apoptotic markers in the tumor cells compared to that in the SEC control group. Caspase-3 and BAX were increased significantly to 4.9-fold and 5.05-fold, respectively, while VEGF and Bcl-2 decreased significantly to 0.47-fold and 0.33-fold, respectively.
RT-PCR gene expression analysis of Caspase3, VEGF, Bcl-2, and BAX in the untreated and treated SEC mice (2 mg/kg BW, SQ). Data were normalized using β-actin as the housekeeping gene. The Red dashed line represents fold change of untreated group = 1. Results are expressed as mean ± SEM (n = 3).*Significant difference between the SEC control and SEC + Smp24 using unpaired t-test (P ≤ 0.05)
Protein Expression of Caspase-3, VEGF, PCNA and Ki67 Using Western Blotting
Western blotting analysis was performed to investigate the apoptotic and antiangiogenic effect of Smp24 peptide. (Fig. 9) shows the effect of Smp24 peptide treatment in the tumor cells compared to that in the SEC control group. The band intensity of Caspase 3 increased significantly to 3.9, while the band intensity of VEGF, PCNA, and Ki67 decreased significantly to 0.36, 0.21, and 0.44, respectively, compared to β-actin.
Protein expression and the band intensities of A Caspase 3. B VEGF. C PCNA. D Ki67 in the untreated and treated SEC mice (2 mg/Kg BW, SQ). Data were normalized using β-actin. Results are expressed as mean ± SEM (n = 3).*Significant difference between the SEC control and SEC + Smp24 using unpaired t-test (P ≤ 0.05)
Discussion
Animal venoms are thought to inhibit adaptive resistance to chemotherapeutic treatments, which is what causes cancer cells to become resistant to treatment. Scorpion toxins work together to kill cancer cells by interfering with their ability to divide, arresting their cell cycle, triggering apoptosis, and activating caspases through various signaling pathways. To determine which venom components are specific to which cancer cells, it is necessary to understand their mode of action and complete characterization (Chatterjee 2018). The ability of the peptide to trigger apoptosis in cancer cells may be connected to its toxicity to cancer cells. Several earlier investigations have found that peptides have necrotizing and apoptotic effects on several cancer cell types. (Panja et al. 2021) Furthermore, the scorpion venom antimicrobial peptide Smp43 was discovered to be essential for the regulation of in vitro breast cancer cell survival, proliferation, cell cycle progression, apoptosis, migration, metastasis, and invasion. The results may inspire new ways of thinking about how to search for and test potential therapeutic methods and targets for the treatment of breast cancer (Teleb et al. 2022).
Hence, the current research characterized cytotoxic and antitumor effects of Smp24 peptide on the solid Ehrlich carcinoma. ELrayess et al. (Elrayess et al. 2020) described the cytotoxicity of Smp24 and Smp43 peptides against two acute leukaemia cell lines. In both tumor cell lines, the cytotoxic actions of Smp24 and Smp43 caused a concentration-dependent decline in cellular ATP rates, culminating in cell death. Smp24 and Smp43 appeared to reduce cell viability in all cell types, but lymphoid and myeloid leukemia cell lines were more susceptible.
In the present study, volumes of solid Ehrlich tumors were reduced in mice treated with Smp24 peptide, tumor growth appeared to be slowed, and the tumor inhibition ratio increased compared to 5-Flurouracil.
The most serious side effects of cancer treatment include myelosuppression and anemia. Reduced RBC counts or hemoglobin levels produce anemia in tumor-bearing mice, which can be caused by hemolytic or myelopathic states. (Mitsuaki et al. 1981) In the present investigation, SEC control mice had lower hemoglobin levels, and RBC counts, but WBC counts increased, whereas Smp24 peptide therapy kept RBCs and WBCs at normal levels, but the amount of hemoglobin decreased, which means that the peptide treatment may cause anemia. The total differential leukocyte count demonstrated that granulocytes increased in SEC-bearing animals, which could be owing to an immediate inflammatory response or pressure caused by SEC cell proliferation. (Hoagland 1982) The Smp24 peptide enhanced the number of granulocytes, which means that the peptide treatment may cause an inflammatory response. Hematological parameters have been investigated, implying that Smp24 may protect the hematopoietic system.
AST and ALT are the two most common liver enzymes in the blood serum. Their serum levels are used to predict a variety of cancers, including pancreatic and breast cancer, as well as liver cell damage and death. (Zhou et al. 2020) Lower albumin and total protein levels were associated with increased ALT and AST enzyme activity, indicating hepatic dysfunction linked to cancer. (Saad et al. 2017) The current study gave the same results. The ALT and AST levels increased, and the total protein and albumin decreased in the SEC-bearing mice, which indicates liver dysfunction and damage due to SEC, while The ALT and AST levels decreased, and the total protein and albumin increased in the Smp24 -treated mice, which indicate that liver functions were returned to their normal state due to treatment with Smp24. (Saad et al. 2017)
Enzymes that fight free radicals SOD and CAT are important scavenging enzymes that remove the H2O2 produced during incomplete oxidation. In biological systems, these antioxidant enzymes have an essential part in the body's defense mechanism against the harmful consequences of free radicals and reactive oxygen species (ROS). (Halliwell and Gutteridge 2015) The activity of CAT and SOD was also reported to be depleted because of tumor progression. (Marklund et al. 1982) The current work with SEC-bearing mice yielded similar results. In comparison to the SEC control group, mice treated with Smp24 peptide had significantly higher levels of SOD, CAT, and GSH in their liver homogenates.
The proliferation index was studied in this paper by comparing the activity of Ki67 in solid tumors from control and Smp24-treated mice. When compared to control mice, the data showed that Smp24 peptide suppressed Ki67 expression significantly in tumor tissues. Ki-67 is a nuclear protein activated in proliferating cells (in all phases of the cell cycle except G0) and is an excellent indicator for cell proliferation. It may also be essential for cell proliferation to continue. (Schlüter et al. 1993; Miller et al. 1994) Numerous scorpion venoms have been shown to inhibit the growth of various cell lines, including human leukemia, prostate cancer, and neuroblastoma. (Das Gupta et al. 2007; Zhang et al. 2009; Zargan et al. 2011) A. crassicauda venom, as a comparison, reduced the growth of human neuroblastoma cell lines by stopping S-phase and inducing apoptosis. (Zargan et al. 2011) Apoptosis is an essential mechanism in response to antitumor drugs. (Makin and Dive 2001; Galeano et al. 2005) For cells undergoing apoptosis, two mechanisms have been identified: (1) mitochondria-mediated apoptosis “intrinsic pathway involving caspase-9” and (2) death-receptor-induced apoptosis “extrinsic pathway involving caspases-8, 10”. (Hu and Kavanagh 2003; Franco et al. 2009) Caspase-3 is a crucial protease that triggers apoptosis by activating caspases-8, which causes DNA damage and cell death. (Zargan et al. 2011) In comparison to their control group, the Smp24 peptide increased caspase-3 expression significantly in solid tumor tissues.
Furthermore, the Smp24 peptide showed an anti-proliferating effect in solid tumors by suppressing the production of VEGF. VEGF promotes tumor cell survival by increasing Bcl-2 expression and preventing tumor cell apoptosis. (Pidgeon et al. 2001) PCNA is a DNA clasp that works as a factor of processivity for DNA polymerase δ and is required for replicating DNA in eukaryotic cells. PCNA is a homotrimer that accomplishes processivity by surrounding DNA and acting as a staircase for proteins involved in DNA replication, DNA repair, chromatin remodeling, and epigenetics to recruit. (Moldovan et al. 2007) The Smp24 peptide significantly reduced the PCNA expression in solid tumor tissues compared to their control group, which suppressed the replication and DNA repair of cancer cells in the peptide-treated group. BAX, also known as Bcl-2-like protein, is a protein produced by the BAX gene in humans. This protein is an apoptotic activator that forms a heterodimer with Bcl-2. The Smp24 peptide significantly increased the BAX expression, which induced apoptosis in the peptide-treated group. Bcl-2 suppresses BAX/BAK oligomerization, which would alternatively cause the mitochondrion to discharge multiple apoptogenic chemicals. Bcl-2 is also known to bind to and inactivate BAX and other pro-apoptotic proteins, preventing them from triggering apoptosis. (Khemtémourian et al. 2006) Accordingly, Smp24 peptide treatment induced apoptosis through caspase-mediated apoptosis and inhibited angiogenesis in the SEC-cancer induced mice, and this validated its anti-cancer activity.
Conclusion
It can be concluded that Smp24 (2 mg/Kg BW, SQ) for 14 days peptide had antiproliferative, apoptotic, and antiangiogenic effects on tumor cells and a decline in tumor volume. Additionally, it demonstrated potential anti-cancer by significantly improving antioxidant properties by increasing antioxidant enzyme levels and inhibiting oxidant characteristics by lowering oxidant parameters. In conclusion, Smp24 is a promising apoptotic and antiangiogenic anti-breast cancer agent with lower side effect toxicity than chemotherapeutic agents. Furthermore, the findings pave the way for further research into the interaction of scorpion venom with anti-cancer medications against breast cancer, like 5-Fluorouracil and other medications.
Data Availability
All data and analyses are available from the corresponding author upon reasonable request.
References
Abdel-Rahman MA, Harrison PL, Strong PN (2015) Snapshots of scorpion venomics. J Arid Environ 112:170–176. https://doi.org/10.1016/j.jaridenv.2014.01.007
Abdel-Rahman MA, Quintero-Hernandez V, Possani LD (2013) Venom proteomic and venomous glands transcriptomic analysis of the Egyptian scorpion Scorpio Maurus Palmatus (Arachnida: Scorpionidae). Toxicon. https://doi.org/10.1016/j.toxicon.2013.08.064
Adam C, Bray TL, Pérez-López AM, Tan EH, Rubio-Ruiz B, Baillache DJ, Houston DR, Salji MJ, Leung HY, Unciti-Broceta A (2022) A 5-FU Precursor designed to evade anabolic and catabolic drug pathways and activated by Pd chemistry in vitro and in vivo. J Med Chem 65(1):552–561. https://doi.org/10.1021/acs.jmedchem.1c01733
Aebi H (1984) [13] Catalase in vitro. In Methods in enzymology; oxygen radicals in biological systems; Academic Press, Vol. 105, pp 121–126. Doi: https://doi.org/10.1016/S0076-6879(84)05016-3.
Almaaytah A, Tarazi S, Mhaidat N, Al-Balas Q, Mukattash TL (2013) Mauriporin, a novel cationic α-helical peptide with selective cytotoxic activity against prostate cancer cell lines from the venom of the scorpion Androctonus Mauritanicus. Int J Pept Res Ther 19(4):281–293. https://doi.org/10.1007/s10989-013-9350-3
Bancroft JD (2008) Theory and Practice of Histological Techniques; Elsevier Health Sciences
Beutler E (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Boraei ATA, Eltamany EH, Ali IAI, Gebriel SM, Nafie MS (2021) Synthesis of new substituted pyridine derivatives as potent anti-liver cancer agents through apoptosis induction: in vitro, in vivo, and in silico integrated approaches. Bioorg Chem 111:104877. https://doi.org/10.1016/j.bioorg.2021.104877
Carneiro BA, El-Deiry WS (2020) Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17(7):395–417. https://doi.org/10.1038/s41571-020-0341-y
Chatterjee B (2018) Animal venoms have potential to treat cancer. Curr Top Med Chem 18(30):2555–2566. https://doi.org/10.2174/1568026619666181221120817
Das Gupta S, Debnath A, Saha A, Giri B, Tripathi G, Vedasiromoni JR, Gomes A, Gomes A (2007) Indian black scorpion (Heterometrus Bengalensis Koch) venom induced antiproliferative and apoptogenic activity against human leukemic cell lines U937 and K562. Leuk Res 31(6):817–825. https://doi.org/10.1016/j.leukres.2006.06.004
Doumas BT, Bayse DD, Carter RJ, Peters T, Jr. Schaffer, R. (1981) A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem 27(10):1642–1650. https://doi.org/10.1093/clinchem/27.10.1642
Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB (2000) Diagnosis and monitoring of hepatic injury i: performance characteristics of laboratory tests. Clin Chem 46(12):2027–2049. https://doi.org/10.1093/clinchem/46.12.2027
Ejaz S, Hashmi FB, Malik WN, Ashraf M, Nasim FH, Iqbal M (2018) Applications of venom proteins as potential anti-cancer agents. Protein Pept Lett 25(7):688–701. https://doi.org/10.2174/0929866524666180614102104
Elrayess RA, Mohallal ME, El-Shahat YM, Ebaid HM, Miller K, Strong PN, Abdel-Rahman MA (2020) Cytotoxic effects of Smp24 and Smp43 scorpion venom antimicrobial peptides on tumour and non-tumour cell lines. Int J Pept Res Ther 26(3):1409–1415. https://doi.org/10.1007/s10989-019-09932-1
Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: ménage à trois. Mutat Res 674(1):3–22. https://doi.org/10.1016/j.mrgentox.2008.11.012
Galeano E, Nieto E, García-Pérez AI, Delgado MD, Pinilla M, Sancho P (2005) Effects of the antitumoural dequalinium on NB4 and K562 human leukemia cell lines: mitochondrial implication in cell death. Leuk Res 29(10):1201–1211. https://doi.org/10.1016/j.leukres.2005.03.014
Ghosh D, Griswold J, Erman M, Pangborn W (2009) Structural Basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457(7226):219–223. https://doi.org/10.1038/nature07614
Guo R, Liu J, Chai J, Gao Y, Abdel-Rahman MA, Xu X (2022) Scorpion peptide Smp24 exhibits a potent antitumor effect on human lung cancer cells by damaging the membrane and cytoskeleton in vivo and in vitro. Toxins 14(7):438. https://doi.org/10.3390/toxins14070438
Hafkenscheid JC, Dijt CC (1979) Determination of serum aminotransferases: activation by pyridoxal-5’-phosphate in relation to substrate concentration. Clin Chem 25(1):55–59. https://doi.org/10.1093/clinchem/25.1.55
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Harlow E, Lane D (1999) Using antibodies: a laboratory manual. CSHL Press, 286
Harrison PL, Abdel-Rahman MA, Strong PN, Tawfik MM, Miller K (2016) Characterisation of three alpha-helical antimicrobial peptides from the venom of Scorpio Maurus Palmatus. Toxicon 117:30–36. https://doi.org/10.1016/j.toxicon.2016.03.014
Hill PG (1985) The measurement of albumin in serum and plasma. Ann Clin Biochem 22(6):565–578. https://doi.org/10.1177/000456328502200604
Hoagland HC (1982) Hematologic complications of cancer chemotherapy. Semin Oncol 9(1):95–102
Hu W, Kavanagh JJ (2003) Anti-cancer therapy targeting the apoptotic pathway. Lancet Oncol 4(12):721–729. https://doi.org/10.1016/S1470-2045(03)01277-4
Ilić IR, Stojanović NM, Radulović NS, Živković VV, Randjelović PJ, Petrović AS, Božić M, Ilić RS (2019) The quantitative ER immunohistochemical analysis in breast cancer: detecting the 3 + 0, 4 + 0, and 5 + 0 allred score cases. Medicina 55(8):461. https://doi.org/10.3390/medicina55080461
Ishak K, Baptista A, Bianchi L, Callea F, Groote JD, Gudat F, Denk H, Desmet V, Korb G, MacSween RNM, Phillips MJ, Portmann BG, Poulsen H, Scheuer PJ, Schmid M, Thaler H (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22(6):696–699. https://doi.org/10.1016/0168-8278(95)80226-6
Jakob C, Liersch T, Meyer W, Becker H, Baretton GB, Aust DE (2008) Predictive value of Ki67 and P53 in locally advanced rectal cancer: correlation with thymidylate synthase and histopathological tumor regression after neoadjuvant 5-FU-based chemoradiotherapy. World J Gastroenterol 14(7):1060–1066. https://doi.org/10.3748/wjg.14.1060
Kendall R, Benoit E, Bogiages J, Bordenkircher R, Caple K, Chen L-L, Cheng T, Hoshino T, Kelley J, Ngo N, Schisano T, Stevenson P, Tsou C, Yang JP (2003) Performance evaluation of the abbott cell-dyn 1800 automated hematology analyzer. Lab Hematol 9(3):143–152
Khemtémourian L, Sani M-A, Bathany K, Gröbner G, Dufourc EJ (2006) Synthesis and secondary structure in membranes of the Bcl-2 anti-apoptotic domain BH4. J Pept Sci 12(1):58–64. https://doi.org/10.1002/psc.686
Makin G, Dive C (2001) Apoptosis and cancer chemotherapy. Trends Cell Biol 11:S22–S26. https://doi.org/10.1016/S0962-8924(01)82111-5
Marklund SL, Westman NG, Lundgren E, Roos G (1982) Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res 42(5):1955–1961
Mikaelian AG, Traboulay E, Zhang XM, Yeritsyan E, Pedersen PL, Ko YH, Matalka KZ (2020) Pleiotropic anti-cancer properties of scorpion venom peptides: Rhopalurus princeps venom as an anti-cancer agent. Drug Des Devel Ther 14:881–893. https://doi.org/10.2147/DDDT.S231008
Miller T, Grogan T, Dahlberg S, Spier C, Braziel R, Banks P, Foucar K, Kjeldsberg C, Levy N, Nathwani B (1994) Prognostic significance of the Ki-67-associated proliferative antigen in aggressive non-Hodgkin’s lymphomas: a prospective southwest oncology group trial. Blood 83(6):1460–1466. https://doi.org/10.1182/blood.V83.6.1460.1460
Mitsuaki M, Ikuo N, Masako H, Yutaka T, Kunio Y (1981) Lipid peroxide levels and lipid content of serum lipoprotein fractions of pregnant subjects with or without pre-eclampsia. Clin Chim Acta 115(2):155–161. https://doi.org/10.1016/0009-8981(81)90071-1
Moldovan G-L, Pfander B, Jentsch S (2007) PCNA, the maestro of the replication fork. Cell 129(4):665–679. https://doi.org/10.1016/j.cell.2007.05.003
Montgomery HAC, Dymock JF ( 1961) Analyst 86: 414
Nafie MS, Arafa K, Sedky NK, Alakhdar AA, Arafa RK (2020) Triaryl dicationic DNA minor-groove binders with antioxidant activity display cytotoxicity and induce apoptosis in breast cancer. Chem Biol Interact 324:109087. https://doi.org/10.1016/j.cbi.2020.109087
Nafie MS, Elghazawy NH, Owf SM, Arafa K, Abdel-Rahman MA, Arafa RK (2022) Control of ER-positive breast cancer by ERα expression inhibition, apoptosis induction, cell cycle arrest using semisynthetic isoeugenol derivatives. Chem Biol Interactions 351:109753. https://doi.org/10.1016/j.cbi.2021.109753
Nafie MS, Khodair AI, Hassan HAY, El-Fadeal NMA, Bogari HA, Elhady SS, Ahmed SA (2021) Evaluation of 2-thioxoimadazolidin-4-one derivatives as potent anti-cancer agents through apoptosis induction and antioxidant activation: in vitro and in vivo approaches. Molecules 27(1):83. https://doi.org/10.3390/molecules27010083
Nguyen T, Guo R, Chai J, Wu J, Liu J, Chen X, Abdel-Rahman MA, Xia H, Xu X (2022) Smp24, a scorpion-venom peptide, exhibits potent antitumor effects against hepatoma HepG2 cells via multi-mechanisms in vivo and in vitro. Toxins 14(10):717. https://doi.org/10.3390/toxins14100717
Nishikimi M, Appaji Rao N, Yagi K (1972) The Occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854. https://doi.org/10.1016/S0006-291X(72)80218-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ortiz E, Gurrola GB, Schwartz EF, Possani LD (2015) Scorpion venom components as potential candidates for drug development. Toxicon 93:125–135. https://doi.org/10.1016/j.toxicon.2014.11.233
Panja K, Buranapraditkun S, Roytrakul S, Kovitvadhi A, Lertwatcharasarakul P, Nakagawa T, Limmanont C, Jaroensong T (2021) Scorpion venom peptide effects on inhibiting proliferation and inducing apoptosis in canine mammary gland tumor cell lines. Animals 11(7):2119. https://doi.org/10.3390/ani11072119
Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ (2001) Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer 85(2):273–278. https://doi.org/10.1054/bjoc.2001.1876
Saad EA, Habib SA, Refai WA, Elfayoumy AA (2017) Malondialdehyde, adiponectin, nitric oxide, c-reactive protein, tumor necrosis factor-alpha and insulin resistance relationships and inter-relationships in type 2 diabetes early stage is metformin alone adequate in this stage. Int J Pharm Pharm Sci 9(10):176. https://doi.org/10.22159/ijpps.2017v9i10.21149
Sarfo-Poku C, Eshun O, Lee KH (2016) Medical application of scorpion venom to breast cancer: a mini-review. Toxicon 122:109–112. https://doi.org/10.1016/j.toxicon.2016.09.005
Schlüter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, Gerdes J (1993) The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol 123(3):513–522. https://doi.org/10.1083/jcb.123.3.513
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 71(3):209–249
Teleb WK, Tantawy MA, Xu X, Hussein AA, Abdel-Rahman MA (2022) Cytotoxicity and molecular alterations induced by scorpion venom antimicrobial peptide smp43 in breast cancer cell lines MDA-MB-231 and MCF-7. Int J Pept Res Ther 29(1):8. https://doi.org/10.1007/s10989-022-10474-2
Uzair B, Bint-E-Irshad S, Khan BA, Azad B, Mahmood T, Rehman MU, Braga VA (2018) Scorpion venom peptides as a potential source for human drug candidates. Protein Pept Lett 25(7):702–708. https://doi.org/10.2174/0929866525666180614114307
Wong JPC, Li B, Kwok HF (2015) Venom peptides and toxins—a prospective spearhead in cancer treatment. Combin Chem High Throughput Screen 20(5):357–375
Zargan J, Sajad M, Umar S, Naime M, Ali S, Khan HA (2011) Scorpion (Androctonus Crassicauda) venom limits growth of transformed cells (SH-SY5Y and MCF-7) by cytotoxicity and cell cycle arrest. Exp Mol Pathol 91(1):447–454. https://doi.org/10.1016/j.yexmp.2011.04.008
Zhang YY, Wu LC, Wang ZP, Wang ZX, Jia Q, Jiang GS, Zhang WD (2009) Anti-proliferation effect of polypeptide extracted from scorpion venom on human prostate cancer cells in vitro. J Clin Med Res 1(1):24–31. https://doi.org/10.4021/jocmr2009.01.1220
Zhou J, He Z, Ma S, Liu R (2020) AST/ALT ratio as a significant predictor of the incidence risk of prostate cancer. Cancer Med 9(15):5672–5677. https://doi.org/10.1002/cam4.3086
Acknowledgements
This study was supported in part by Science, Technology & Innovation Funding Authority (STDF) under grant number 45890 and the Academy of Scientific Research and Technology (ASRT, Egypt; China- Egypt Scientific and Technological Cooperation Program) to Mohamed A. Abdel-Rahman and the Chinese National Natural Science Foundation (31861143050) to Xueqing Xu.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MSN and MAR designed the idea of the study, BSF and MSN carried out the biological assays under supervision of IAI and MAR while LMF carried out the histopathological examination. BSF and MSN analyzed data, made visualization, and wrote the original draft with the literature review. XX provided the peptide Smp24. All authors agreed to the final manuscript form.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical Approval
This study was agreed by the Research Ethics Committee, Faculty of Science, Suez Canal University. Reference No: REC-10-2020.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fawzy, B.S., Nafie, M.S., Ali, I.A.I. et al. Scorpion Venom Peptide Smp24 Revealed Apoptotic and Antiangiogenic Activities in Solid-Ehrlich Carcinoma Bearing Mice. Int J Pept Res Ther 29, 29 (2023). https://doi.org/10.1007/s10989-023-10494-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-023-10494-6