Abstract
Human GLP-1 (glucagon-like peptide-1) can produce a remarkable improvement in glycemic control in patients with type 2 diabetes. However, its clinical benefits are limited by its short half-life, which is less than 2 min because of its small size and rapid enzymatic inactivation by dipeptidyl peptidase IV. We engineered Exendin-4-Fc, a 66-kDa fusion protein by linking an IgG2 Fc to Exendin-4. A stably transfected Chinese hamster ovary cell line was obtained using electroporation. Exendin-4-Fc stimulated insulin secretion in INS-1 cells in a dose- and glucose-dependent manner and increased insulin mRNA expression. The plasma half-life of Exendin-4-Fc in cynomolgus monkeys was approximately 133.92 ± 25.1 h. In the KKAy mouse model of diabetes, one intraperitoneal injection of Exendin-4-Fc (1 mg/kg) reduced blood glucose levels for 5 days. A 4-week repeat-administration study identified sustained effects on blood glucose levels. Oral glucose tolerance tests conducted at the beginning and end of this 4-week period showed that Exendin-4-Fc produced a stable glucose lowering effect. In addition, KKAy mice treated with Exendin-4-Fc showed statistically significant weight loss from day 23. In conclusion, these properties of Exendin-4-Fc demonstrated that it could be a potential long-acting GLP-1 receptor agonist for the treatment of type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diabetes is a global concerned disease characterized with hyperglycemia. According to the International Diabetes Federation, there are currently 425 million diabetic patients worldwide, and it is estimated that by 2045, there will be nearly 700 million diabetic patients. Type 2 diabetes mellitus (T2DM) is a progressive chronic disease with insulin resistance and β cell failure which occupies 90% of total diabetes. T2DM is usually caused by unhealthy lifestyle and/or genetic factors. It can cause a variety of serious complications, including diabetic ketoacidosis, non-ketotic hyperosmolar coma, heart disease, stroke, chronic renal failure, foot ulcers and eye lesions. The classic drug for T2DM treatment is insulin. However, the frequent injection, drug resistance, adverse effects limits its application.
In recent years, the glucagon-like peptide-1 (GLP-1) is considered to be an ideal drug for T2DM treatment. GLP-1 is a glucose-dependent intestinal hypoglycemic polypeptide hormone secreted by L cells of the terminal jejunum, ileum and colon. The peptide hormone promotes glycemic-dependent insulin secretion, inhibits glucagon secretion, suppresses appetite, and stimulates pancreatic β cell proliferation and inhibits its apoptosis. However, the natural GLP-1 half-life is less than 2 min in plasma because of its rapid enzymatic degradation by DPP-IV. Extension of the t1/2 has become a key issue for research relating to GLP-1. DPP-IV inhibitors (sitagliptin, vildagliptin, saxagliptin, alogliptin, and linagliptin) and GLP-1 receptor agonists have therefore been developed to extend the t1/2. Clinical research confirmed that the use of DPP-IV inhibitors or GLP-1 receptor agonists significantly reduced fasting and postprandial blood glucose, HbA1c, and β-cell function. DPP-IV inhibitors and GLP-1 receptor agonists are mainly used to treat poorly controlled T2DM after sulfonylureas or thiazolidinediones have been employed. Their use is associated with a lower incidence of hypoglycemic events, and with good clinical safety and tolerability (Light et al. 2002; Baggio and Drucker 2007; Pratley and Gilbert 2008; Murage et al. 2010; Aroda et al. 2012).
It is worth noting that more than 80% of people with T2DM are overweight or obese. There is robust research evidence indicating that obesity is a key determinant of insulin secretion and resistance to the effects of insulin. A modest reduction in body weight may therefore be beneficial for hyperglycemic control. According to the new guidelines of the American Diabetes Association and the European Association for the Study of Diabetes, GLP-1 receptor agonists are the only commonly used anti-diabetic agents shown to reduce body weight (Inzucchi et al. 2012).
Five novel GLP-1 receptor agonists (Exenatide, liraglutide, albiglutide, semaglutide, and dulaglutide) have been approved for the treatment of T2DM. Structural modification mainly includes cleavage site replacement (Siegel et al. 1999) and the addition of a macromolecular protein or an aliphatic chain (Huang et al. 2008; Lorenz et al. 2013). These molecules can retain the biological activities of GLP-1, while showing a prolonged t1/2 from about 2 minutes to hours, even days (Lorenz et al. 2013). The use of GLP-1 receptor agonists can cause gastrointestinal reactions and thyroid C cell proliferation (Astrup et al. 2012; Macconell et al. 2012). There is no research evidence for a correlation between these adverse reactions and the t1/2. However, antibodies and allergic reactions are frequently reported by research studies involving GLP-1 receptor agonists (Russell-Jones 2010; Buse et al. 2011). To reduce immunogenicity and prolong t1/2, Exendin-4-Fc was constructed by fusing the Exendin-4 with IgG2 Fc. The Fc region of Exendin-4 has a t1/2 of 16 days (Vafa et al. 2014). This longer t1/2 is based on reduced renal clearance and FcRN-mediated receptor recycling (Lobo et al. 2004). In pre-clinical studies, Exendin-4 showed minimal binding to Fcγ Rs and activation of immune responses, as compared to other previously well-characterized ‘muted’ Fc variants, including aglycosylated IgG1, IgG2m4, and IgG4 (Vafa et al. 2014). The Exendin-4-Fc fusion protein was therefore predicted to show a longer t1/2 and lower immune activity. We expressed the Exendin-4-Fc fusion protein and tested its bioactivity both in vitro and in vivo in order to investigate whether this represented a potential long-acting GLP-1 receptor agonist for the treatment of T2DM.
Materials and Methods
Materials
Cell culture medium and fetal bovine serum were purchased from Gibco (Beijing, China). The enzyme-linked immunosorbent assay (ELISA) kits for insulin were purchased from NEObioscience (Beijing, China). All other reagents, unless otherwise indicated, were purchased from Sigma-Aldrich (St Louis, MO, USA).
Animals and Ethics Statement
This study was approved by the Institutional Animal Care and Use Committee at the Animal Center, Beijing Eastern Biotech, Co., Ltd. (ACU11-527). Six of cynomolgus monkeys aged 2 years old (2.42–2.61 kg; animal numbers 1009548, 1202501, and 1202473) were obtained from the Fuze Wild Animal Co. Ltd. Center in Nanning. The monkeys were maintained in stainless steel cages (L × W × H: 1000 × 1000 × 1000 mm) at a temperature of 20 ± 2 °C, with 40–70% relative humidity, a 12-h light–dark cycle with artificial illumination from 07:00 to 19:00, and a room air exchange rate of 12 times/h. Animals were fed with fresh supplies of 100 g of standard monkey diet and 50 g of fruit and vegetables twice daily. All monkeys were allowed to socialize by being housed in pairs during the day from approximately 09:00 to 15:00. Seasonal produce, seeds, and cereal were offered as supplements for environmental enrichment. To minimize suffering, monkeys were anesthetized with 10 mg/kg ketamine and 20 mg/kg pentobarbital sodium. The monkeys were evaluated by a veterinary surgeon to determine whether the procedure should be carried out or discontinued. Since no significant trauma was involved in this study, only a brief muscle twitch (restricted to the stimulated limb) was expected.
Male KKAy mice (10 weeks old) were purchased from Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences and maintained in a controlled environment (20 ± 2 °C, 50–60% humidity) with a 12-h light–dark cycle (lights on at 07:00 and off at 19:00) and free access to water and food. During this study, blood samples were obtained by tail-vein pricking. This method involves only slight trauma and was selected to minimize the potential suffering of these mice (Chung et al. 2011). The procedure would have been discontinued if the mice became stressed. All manipulations were performed under analgesia (2% inhaled isoflurane) to minimize suffering. Eighteen KKAy mice were used in this study and none of these became ill or died prior to its conclusion. At the end of the experiment, mice were euthanized by CO2 asphyxiation.
All animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize animal suffering and to reduce the number of animals used.
Cell Culture
INS-1 cells were obtained from the ATCC and maintained in RPMI 1640 medium containing 10% of FBS (Gibco, USA) at 37 °C in an atmosphere of humidified air (95%) and CO2 (5%).
Plasmid Construction
A sequence encoding Exendin-4-Fc was designed using the nucleotide sequences and synthesized. This sequence was then digested by the restriction enzymes, HindIII and EcoRI (New England Biolabs, Ipswich, MA, USA), and inserted into the mammalian SGLs expression vector (obtained from the Research Center of Pharmacokinetics, Beijing, China). The recombinant SGLs-Exendin-4-Fc plasmid was verified by DNA sequencing (forward primer: 5ʹ-CAGGACCACGTCGTGCCAGT-3ʹ).
Expression and Purification of Exendin-4-Fc
Chinese hamster ovary cells (passage 13–19) were obtained from the China Infrastructure of Cell Line Resources and expanded in CD-CHO complete medium (Invitrogen, Carlsbad, CA, USA) containing 8 mM glutamine. Stably expressing clones were obtained after electroporation with the SGLs-Exendin-4-Fc plasmid (Gene Pulser Xcell Electroporation System; BioRad, CA, USA). Highly expressing clones were selected based on SDS-PAGE and ELISA analysis using a horseradish peroxidase (HRP)-conjugated goat anti-human IgG monoclonal antibody at a 1:5000 dilution (catalog number 31413; Pierce, Rockford, IL, USA). Stable cell lines were cultured continuously for 12 days with rotation at 225 rpm at 37 °C. The expression medium was then harvested and filtered. The Exendin-4-Fc fusion protein was purified from the medium by protein A affinity chromatography (Hi-Trap protein A column; GE Healthcare, Piscataway, NJ, USA). The retained protein was washed with 10 mM phosphate-buffered saline (PBS; 1 mL/min flow rate) and eluted with 100 mM sodium citrate-buffered saline (pH 3.0). All of these purification steps were carried out at 4 °C. The sodium citrate-buffered saline was removed using an Amicon Ultra-4 ultrafiltration tube with a molecular weight cut-off of 10,000 Da (Millipore, Bedford, MA, USA) at 4500 × g and 4 °C for 45 min. The products were characterized by high-performance liquid chromatography, quantified using a bicinchoninic acid protein assay kit, and stored in 10 mM PBS (pH 7.4) at − 80 °C.
Glucose-Induced Insulin Secretion
INS-1 cells were plated in 96-well assay plates at a concentration of 5 × 104 cells/well. After a 2-day maintenance period, 200 μL RPMI 1640 without glucose was added for 120 min to precondition the cells. This medium was then replaced with 200 μL RPMI 1640 supplemented with 2.2 mM or 16.8 mM glucose and Exendin-4-Fc (1 nM, 10 nM, or 100 nM) for 2 h. The amount of insulin released into the media was then evaluated using a rat ultrasensitive insulin ELISA kit.
Glucose-Induced mRNA Synthesis of Insulin
Total RNA was also isolated from INS-1 cells incubated with Exendin-4-Fc using Trizol. For cDNA synthesis, 0.5 μg of total RNA was reverse-transcribed using a RevertAid First Strand cDNA Synthesis Kit (Thermo, USA). Quantitative PCR (qPCR) was performed using a KAPA SYBR® FAST qPCR Kit and the fluorescent signal was detected by a Roche LightCycler® 96 system (Sweden) (Buteau et al. 1999). The following oligonucleotide primer pairs (forward and reverse) were used to amplify rat insulin: 5ʹ-CACCCAAGTCCCGTCGTGAAGT-3ʹ and 5ʹ-GATCCACAATGCCACGCTTCTG-3ʹ.
Pharmacokinetics in Cynomolgus Monkeys
Pharmacokinetic studies of Exendin-4-Fc were carried out in adult male cynomolgus monkeys (n = 6). The monkeys were lightly anesthetized with 5% inhaled isoflurane, weighed, and placed on a heated primate chair to maintain normal body temperature. The heart rate, respiratory rate, blood pressure, and breathing pattern were continuously monitored. After fixation of the foreleg, blood (0.6 mL) was collected from the foreleg vein immediately pre-administration (time 0), and at 2, 4, 8, 12, 48, 72, 96, 192, 240, and 288 h after a single subcutaneous injection of Exendin-4-Fc (0.1 mg/kg) using a retained needle and a 1-mL syringe. After the collection of blood, the animal was returned to the recovery cage and monitored until it was able to sit up. Plasma samples were obtained by centrifugation and stored at − 70 °C in polyethylene tubes containing 10 μL of DPP-IV inhibitor (Millipore, Milford, MA, USA). The Exendin-4-Fc levels were determined by sandwich ELISA, using a mouse anti-GLP-1 monoclonal antibody (1:500; catalog number ab121086; Abcam, Cambridge, UK), a secondary HRP-conjugated goat anti-human IgG monoclonal antibody (1:20,000; Pierce), and TMB as the chromogenic reagent. Standard curves were prepared for Exendin-4-Fc in cynomolgus monkey plasma. The ELISA assay range was approximately 0.66–1000 ng/mL. Concentrations of Exendin-4-Fc were calculated using Watson LIMS v.7.3.0.01 (Thermo Scientific, Inc.). Pharmacokinetic data were analyzed by noncompartmental methods using WinNonLin version 5.2.1 (Pharsight, Inc., Mountain View, CA, USA).
Oral Glucose Tolerance Test (OGTT)
Eighteen KKAy mice (10 weeks old; 34–37 g) were acclimated for 3 days and fasted overnight (12 h) before they were randomly divided into three groups (n = 6/group).These groups received Exendin-4-Fc (1 mg/kg), Exenatide (1 mg/kg), or saline by intraperitoneal injection 30 min prior to the OGTT. Glucose was dissolved in saline and administered by oral gavage at a dose of 2 g/kg. Blood samples were obtained by tail-vein pricking at pre-determined time points (− 30, 0, 10, 20, 30, 60, and 90 min) and glucose levels were measured using a glucose meter (One Touch®Ultra; LifeScan, NJ, USA) (Amiram et al. 2013).
Acute and Long-Term Pharmacodynamics
After a 3-day acclimatization period, 10-week-old male KKAy mice were injected intraperitoneally with Exendin-4-Fc (1 mg/kg), Exenatide (1 mg/kg), or saline (200 μL). Blood glucose levels were measured using a glucose meter at 0, 1, 2, 3, 4, 5, 6, and 7 days (08:00).
For the long-term study, KKAy were acclimatized for 3 days prior to intraperitoneal injection of Exendin-4-Fc (1 mg/kg), Exenatide (1 mg/kg), or saline (200 μL) once every 3 days for 4 weeks. Blood glucose was measured using a glucose meter before these injections. Body weight and food intake were recorded every day. At the end of this treatment period, the mice had 10 drug administration-free days before they were subjected to the OGTT test described above. Blood samples were obtained by tail-vein pricking at pre-determined time points (− 30, 0, 10, 20, 30, 60, and 90 min) and glucose levels were measured using a glucose meter. Blood samples (obtained at − 30 and 30 min) were centrifuged at 3000 rpm for 5 min at 4 °C to isolate plasma. Insulin levels were evaluated using a mouse ultrasensitive insulin ELISA kit (Alpco, Beijing, China).
Data Analysis
Concentrations of Exendin-4-Fc and insulin were calculated using a four-parameter algorithm. The area under the blood glucose concentration–time curve (AUC) in the OGTT was calculated using the following algorithm: AUC (mM 120 min) = (BG-30 + BG0) × 15 + (BG0 + BG10) × 5 + (BG10 + BG20) × 5 + (BG20 + BG30) × 5 + (BG30 + BG60) × 15 + (BG60 + BG90) × 15. BG-30, BG0, BG10, BG20, BG30, BG60, and BG90 represent the blood glucose levels at − 30, 0, 10, 20, 30, 60, and 90 min, respectively. All data were expressed as means ± the standard error of the mean (SEM), and differences between groups in assays were determined by one-way ANOVA followed by Tukey’s post hoc multiple comparison test using GraphPad Prism 5 software. A p-value of less than 0.05 was regarded as statistically significant.
Results
Construction, Expression, and Purification of Exendin-4-Fc
DNA encoding Exendin-4-Fc was synthesized and inserted into the mammalian SGLs expression vector. DNA sequencing demonstrated that the insertion sequence was correct. The IgG2 Fc included a DPP-IV-protected linked to the Exendin-4 by a peptide linker (GGGGAGGGG) (Hashimoto et al. 2000). PCR results showed the DNA length was nearly 1000 bp and SDS-PAGE analysis confirmed that the apparent molecular weight of the protein was 66 kDa (Fig. 1). After one-step purification using protein A affinity chromatography, this expressed Exendin-4-Fc was used in subsequent experiments.
In Vitro Activity of Exendin-4-Fc
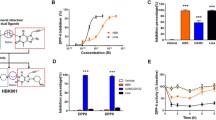
Tests of glucose-induced insulin secretion were performed using INS-1 cells exposed to various concentrations of Exendin-4-Fc. As shown in Fig. 2A, Exendin-4-Fc stimulated insulin secretion in a glucose-dependent manner. In cells incubated with medium containing 2.8 mM glucose, there were no statistically significant differences between each group. However, cells incubated with medium containing 16.8 mM glucose secreted significantly more insulin in the presence of 10 nM (5.2 ± 0.4 ng/mL) and 100 nM (7.3 ± 0.3 ng/mL) Exendin-4-Fc, as compared with control cells (2.5 ± 0.5 ng/mL, p < 0.05). To investigate this effect on insulin synthesis further, mRNA was isolated from the INS-1 cells exposed to 16.8 or 2.8 mM glucose and 100 nM Exendin-4-Fc and insulin mRNA expression was evaluated by qPCR (Fig. 2B). Cells exposed to 16.8 mM glucose in the presence of 100 nM Exendin-4-Fc showed a significant increase in insulin mRNA level, as compared to those without Exendin-4-Fc, while cells exposed to 2.8 mM glucose only showed no significant change in insulin mRNA level with or without Exendin-4-Fc.
The in vitro activity of Exendin-4-Fc in inducing insulin synthesis and secretion. A Exendin-4-Fc could not induce insulin secretion at the low-glucose condition. However, in the high-glucose condition, 10 mM and 100 mM of Exendin-4-Fc could significantly increase the secretion of insulin. B The qPCR results showed the insulin mRNA levels in the INS-1 cells was significantly upregulated when treated with 100 mM Exendin-4-Fc and 16.8 mM glucose. *p < 0.05
Exendin-4-Fc Has an Extended t1/2 and Produced Sustained Glucose Reduction
The pharmacokinetics of Exendin-4-Fc were studied in cynomolgus monkeys after a single dose of 0.01 mg/kg or 0.1 mg/kg. The t1/2 of Exendin-4-Fc was 133.92 ± 25.1 h (0.1 mg/kg) and it could be detected for more than 12 days after a single injection (Fig. 3).
The blood glucose level in KKAy mice showed a sustained reduction after a single dose of 1 mg/kg Exendin-4-Fc (Fig. 4). Mice treated with Exendin-4-Fc showed significantly lower levels of postprandial glucose from days 1 to 5, as compared to the vehicle group. The Exenatide-treated group showed a statistically significant hypoglycemic effect only on the first 2 days post-administration. Based on this result and on pre-clinical research involving dulaglutide, the administration frequency was set to 3 days (Glaesner et al. 2010).
Exendin-4-Fc Reduced Postprandial Glucose and Body Weight in KKAy Mice
KKAy mice were treated with intraperitoneal Exendin-4-Fc once every 3 days for 4 weeks. During this period, Exendin-4-Fc significantly reduced the plasma glucose levels (Fig. 5A). The AUC from days 1 to 28 was significantly larger in the vehicle-treated group than in the 1 mg/kg Exendin-4-Fc-treated group (p < 0.001; Fig. 5B). KKAy mice treated with Exendin-4-Fc had a significantly lower body weight from day 23, while the group treated with Exenatide showed no significant difference, as compared to the vehicle group (Fig. 5C). The vehicle group consumed ~ 1.5-fold more food than the Exendin-4-Fc group and ~ 1.3-fold more food than the Exenatide group. In addition, the Exendin-4-Fc group consumed significantly less food than the Exenatide group.
Long-term pharmacodynamics data in KKAy mice. A Blood glucose was measured prior to the administration of the indicated treatments to KKAy mice every 3 days for 4 weeks. B The area under the curve (AUC) of the data presented in A. C Mouse body weights were recorded every day. D Food intake of each group. *p < 0.05; **p < 0.01; ***p < 0.001
OGTT tests were performed in KKAy mice at the beginning and end (10 days after the last dose) of a 4-week treatment with Exendin-4-Fc, Exenatide, or saline to investigate the effects on postprandial glucose levels. Compared with the vehicle group, mice treated with either Exenatide or Exendin-4-Fc showed reduced glucose levels. Calculation of the AUC showed that mice treated with Exenatide showed a reduction of ~ 61.5% before treatment and of ~ 24.9% after treatment. Exendin-4-Fc produced a greater hypoglycemic effect after treatment, with a reduction of ~ 31.2% before treatment and ~ 43.5% after treatment (Fig. 6A, B). Insulin levels were tested after 4 weeks of treatment to further investigate the effect of GLP-1 fusion proteins in KKAy mice. The groups treated with Exenatide and Exendin-4-Fc showed no significant improvement in basal insulin levels prior to gastric perfusion of glucose. As compared with vehicle-treated mice, those treated with Exenatide or Exendin-4-Fc showed significant improvements in insulin secretion at 30 min (Fig. 6C).
OGTT in KKAy mice. A OGTT in KKAy mice at the beginning of the 4-week treatment period. Glucose (2 g/kg) was administered 30 min before injection of Exendin-4-Fc (1 mg/kg), Exenatide (1 mg/kg), or saline (200 μL). The area under the curve (AUC) for the glucose levels is shown in the upper left panel. B OGTT in KKAy mice at the end of the 4-week treatment period. The AUC for the glucose levels is shown in the upper left panel. C Insulin levels were tested at 0 and 30 min during the OGTT test*p < 0.05; **p < 0.01; ***p < 0.001
Discussion
Exendin-4-Fc fusion proteins can be used to produce molecules that retain the glycemic control properties of GLP-1, with longer t1/2 values. We successfully engineered Exendin-4-Fc, a 68-kDa fusion protein linking IgG2 Fc with Exendin-4 protein. We expressed this in a eukaryotic expression system because this is the only established system that is suitable for the expression of complex recombinant proteins with human-like glycoforms that are bioactive in humans (Maccani et al. 2014).
Consistent with our previous observations using native GLP-1 and GLP-1 receptor agonists, incubation of INS-1 cells with Exendin-4-Fc resulted in concentration-dependent effects on insulin release (MacDonald et al. 2002). Further mRNA analyses of these cells indicated that Exendin-4-Fc also increased insulin synthesis. This confirmed that the method used to purify the expressed Exendin-4-Fc did not inhibit its bioactivity. The results also confirmed that Exendin-4-Fc stimulated insulin secretion in a glucose-dependent manner.
Exenatide (comprising native GLP-1 fused with IgG4) was used as a positive control in the present in vivo study. The t1/2 of Exenatide in cynomolgus monkeys was approximately 37.5 ± 4.9 h (data not shown), while the t1/2 of Exendin-4-Fc was 57.1 ± 4.5 h. This represented a significant improvement over native GLP-1 (t1/2 < 5 min). Other GLP-1 agonists have shown t1/2 values of 51.6 ± 3.2 h in monkeys (dulaglutide), 11–13 h in humans (liraglutide), and 2.4 h in humans (Exenatide BID). Due to species differences, dulaglutide (a human GLP-1 variant fused with a human IgG4 variant) showed a longer t1/2 in humans than in monkeys (120 h versus 51.6 ± 3.2 h, respectively) and further research will be required to determine whether Exendin-4-Fc also has a longer t1/2 in humans.
GLP-1 and GLP-1 receptor agonists improve glycemic control in T2DM by reducing postprandial glucose levels (Meier 2012). The present study used 10-week-old male KKAy mice as an in vivo model of T2DM because these mice develop non-insulin-dependent diabetes mellitus spontaneously at the age of 12 weeks (Liu et al. 2010; Hou et al. 2013; Zhang et al. 2013). Although KKAy, db/db, and ob/ob mice are all used as genetic mouse models of T2DM, KKAy mice were the least expensive of these and were readily available. Exendin-4-Fc produced a more sustained hypoglycemic effect in KKAy mice than did Exenatide. An intraperitoneal injection of Exendin-4-Fc (1 mg/kg) reduced blood glucose levels for 5 days, while Exenatide affected this for 2 days. Based on this observation and on previous studies of dulaglutide, we elected to administer these treatments once every 3 days during the present long-term pharmacodynamics study. Our findings suggested that Exendin-4-Fc produced a stable hypoglycemic and insulin secretion effect during the treatment period. Notably, the drug resistance observed in animals treated with Exenatide was not seen in those treated with Exendin-4-Fc. Bioactivity is often reduced by neutralizing antibodies, which can be generated at different levels, depending on the immunogenicity of the protein drug (Rossman 2004). Several studies of GLP-1 receptor agonists have reported the formation of antibodies and Exenatide treatment was associated with antibody generation in 74% of patients (Drucker et al. 2008). Liraglutide, which shares 97% of its amino acid sequence with the endogenous human protein, also generated antibodies in 4–13% of patients (Russell-Jones et al. 2009; Zinman et al. 2009). The variation present in IgG2 completely eliminates its affinity for FcγRs and the C1q complement protein, which induce antibody-dependent cellular cytotoxicity and phagocytosis, as well as complement-dependent cytotoxicity. These ‘silent’ properties of Exendin-4 render it superior to IgG4 for use in therapeutic non-immunostimulatory fusion proteins. In addition, the GLP-1 variant used in the present study is a close structural homolog of native human GLP-1, with three amino acid substitutions (A8G/G26E/R36G) that confer protection from DPP-IV cleavage. The longer t1/2 and stable glucose-lowering effects observed in our in vivo study indicated that Exendin-4-Fc did not trigger a significant level of antibody production and was not metabolized rapidly. This suggested that Exendin-4-Fc may be appropriate for therapeutic application as a GLP-1 receptor agonist.
Their effect on weight loss is another important property of GLP-1 receptor agonists (Mayo et al. 2003). In the present pharmacodynamic study, 4-week treatment with Exendin-4-Fc resulted in significant weight loss from day 23, as compared to the vehicle group. The food intake result also supported this conclusion and indicated that Exendin-4-Fc may reduce weight by inhibiting feeding. However, it is not possible to extrapolate these pre-clinical findings to infer effects in humans. For example, dulaglutide includes the same GLP-1 variant as Exendin-4-Fc and produces significant weight reduction in db/db mice, but not in humans.
In conclusion, the hypoglycemic effects of Exendin-4-Fc were sustained for longer than those of Exenatide in KKAy mice. Pharmacokinetic and pharmacodynamic analyses of Exendin-4-Fc confirmed that it had a prolonged t1/2 and could reduce body weight. These superior features indicated that Exendin-4-Fc could provide a potential long-acting GLP-1 receptor agonist for the treatment of T2DM.
Data Availability
All data generated or analysed during this study are included in this published article.
Code availability
Not applicable.
Consent for Publication
Not applicable.
References
Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A (2013) A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. J Control Release 172:144–151. https://doi.org/10.1016/j.jconrel.2013.07.021
Aroda VR, Henry RR, Han J, Huang W, DeYoung MB, Darsow T, Hoogwerf BJ (2012) Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther 34:1247-1258.e1222. https://doi.org/10.1016/j.clinthera.2012.04.013
Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, Savolainen MJ, Van Gaal L, Investigators NN (2012) Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 36:843–854. https://doi.org/10.1038/ijo.2011.158
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157. https://doi.org/10.1053/j.gastro.2007.03.054
Buse JB, Garber A, Rosenstock J, Schmidt WE, Brett JH, Videbaek N, Holst J, Nauck M (2011) Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. J Clin Endocrinol Metab 96:1695–1702. https://doi.org/10.1210/jc.2010-2822
Buteau J, Roduit R, Susini S, Prentki M (1999) Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 42:856–864. https://doi.org/10.1007/s001250051238
Chung HS, Oh JY, Yoo SB, Lee SM, Cho HS (2011) The N-terminal alanine-extended GLP-1/IgG-Fc fusion protein confers resistance to DPP-IV and reduces serum glucose level in db/db mice. Regul Pept 170:1–3. https://doi.org/10.1016/j.regpep.2011.05.002
Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L, D.-S. Group (2008) Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372:1240–1250. https://doi.org/10.1016/S0140-6736(08)61206-4
Glaesner W, Vick AM, Millican R, Ellis B, Tschang SH, Tian Y, Bokvist K, Brenner M, Koester A, Porksen N, Etgen G, Bumol T (2010) Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev 26:287–296. https://doi.org/10.1002/dmrr.1080
Hashimoto Y, Ikenaga T, Tanigawa K, Ueda T, Ezak I, Imoto T (2000) Expression and characterization of human rheumatoid factor single-chain Fv. Biol Pharm Bull 23:941–945. https://doi.org/10.1248/bpb.23.941
Hou GJ, Li CN, Liu SN, Huan Y, Liu Q, Sun SJ, Li LY, Hou SC, Shen ZF (2013) Long-term treatment with EXf, a peptide analog of Exendin-4, improves beta-cell function and survival in diabetic KKAy mice. Peptides 40:123–132. https://doi.org/10.1016/j.peptides.2013.01.010
Huang YS, Chen Z, Chen YQ, Ma GC, Shan JF, Liu W, Zhou LF (2008) Preparation and characterization of a novel Exendin-4 human serum albumin fusion protein expressed in Pichia pastoris. J Pept Sci 14:588–595. https://doi.org/10.1002/psc.942
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR, American Diabetes Association and European Association for the Study of Diabetes (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35:1364–1379. https://doi.org/10.2337/dc12-0413
Light PE, Manning Fox JE, Riedel MJ, Wheeler MB (2002) Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol 16:2135–2144. https://doi.org/10.1210/me.2002-0084
Liu M, Wu K, Mao X, Wu Y, Ouyang J (2010) Astragalus polysaccharide improves insulin sensitivity in KKAy mice: regulation of PKB/GLUT4 signaling in skeletal muscle. J Ethnopharmacol 127:32–37. https://doi.org/10.1016/j.jep.2009.09.055
Lobo ED, Hansen RJ, Balthasar JP (2004) Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 93:2645–2668. https://doi.org/10.1002/jps.20178
Lorenz M, Evers A, Wagner M (2013) Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorg Med Chem Lett 23:4011–4018. https://doi.org/10.1016/j.bmcl.2013.05.022
Maccani A, Landes N, Stadlmayr G, Maresch D, Leitner C, Maurer M, Gasser B, Ernst W, Kunert R, Mattanovich D (2014) Pichia pastoris secretes recombinant proteins less efficiently than Chinese hamster ovary cells but allows higher space–time yields for less complex proteins. Biotechnol J 9:526–537. https://doi.org/10.1002/biot.201300305
Macconell L, Brown C, Gurney K, Han J (2012) Safety and tolerability of Exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 5:29–41. https://doi.org/10.2147/DMSO.S28387
MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB (2002) The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51(Suppl 3):S434-442. https://doi.org/10.2337/diabetes.51.2007.s434
Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ (2003) International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev 55:167–194. https://doi.org/10.1124/pr.55.1.6
Meier JJ (2012) GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 8:728–742. https://doi.org/10.1038/nrendo.2012.140
Murage EN, Gao G, Bisello A, Ahn JM (2010) Development of potent glucagon-like peptide-1 agonists with high enzyme stability via introduction of multiple lactam bridges. J Med Chem 53:6412–6420. https://doi.org/10.1021/jm100602m
Pratley RE, Gilbert M (2008) Targeting incretins in type 2 diabetes: role of GLP-1 receptor agonists and DPP-4 inhibitors. Rev Diabet Stud 5:73–94. https://doi.org/10.1900/RDS.2008.5.73
Rossman HS (2004) Neutralizing antibodies to multiple sclerosis treatments. J Manag Care Pharm 10:S12-19
Russell-Jones D (2010) The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int J Clin Pract 64:1402–1414. https://doi.org/10.1111/j.1742-1241.2010.02465.x
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R, Liraglutide E, S.U.S.G. Action in Diabetes 5 met (2009) Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 52:2046–2055. https://doi.org/10.1007/s00125-009-1472-y
Siegel EG, Gallwitz B, Scharf G, Mentlein R, Morys-Wortmann C, Folsch UR, Schrezenmeir J, Drescher K, Schmidt WE (1999) Biological activity of GLP-1-analogues with N-terminal modifications. Regul Pept 79:93–102. https://doi.org/10.1016/s0167-0115(98)00155-4
Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR (2014) An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 65:114–126. https://doi.org/10.1016/j.ymeth.2013.06.035
Zhang J, Xue C, Zhu T, Vivekanandan A, Pennathur S, Ma ZA, Chen YE (2013) A tripeptide Diapin effectively lowers blood glucose levels in male type 2 diabetes mice by increasing blood levels of insulin and GLP-1. PLoS ONE 8:e83509. https://doi.org/10.1371/journal.pone.0083509
Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L, Investigators L-S (2009) Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 32:1224–1230. https://doi.org/10.2337/dc08-2124
Funding
This study is supported by the company’s own funding.
Author information
Authors and Affiliations
Contributions
WZ reviewed the literature, managed the experiments and wrote the manuscript. ML reviewed and revised the manuscript. YZ reviewed the manuscript. YB supervised the study and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Exendin-4-Fc has been developed as a drug (under name JY09) for type 2 diabetes and a Phase II clinical trial (#CTR20192166) is being carried out in China. Interest of Exendin-4-Fc is protected by Patent CN101891823B.
Ethical Approval
This study was approved by the Institutional Animal Care and Use Committee at the Animal Center, Beijing Eastern Biotech, Co., Ltd. (ACU11-527).
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, W., Li, M., Zan, Y. et al. Expression and Characterization of a Potent Long-Acting GLP-1 Receptor Like Agonist, Exendin-4-Fc. Int J Pept Res Ther 27, 2517–2526 (2021). https://doi.org/10.1007/s10989-021-10269-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-021-10269-x