Abstract
Context
The alfalfa weevil Hypera postica Gyllenhal (Coleoptera: Curculionidae) is one of the most destructive pests of alfalfa worldwide. Both local and landscape-scale factors can significantly influence crop pests, natural enemies, and the effectiveness of biological control services, but the relative influence of these factors is unclear.
Objectives
We investigated the influence of the local variables and surrounding landscape composition and configuration on the abundance of alfalfa weevil, and on the abundance and parasitism rates of its larval parasitoids, Bathyplectes spp.
Methods
We sampled 65 commercial alfalfa fields along the Ebro Basin, Spain, over a period of 3 years, recording the field characteristics and landscape structure at three buffer radii of 250, 500 and 1000 m from the center of each field.
Results
The abundance of weevil larvae was positively associated with the field perimeter and with the uncut alfalfa surrounding the pipes of the sprinkler irrigation system, but only one configuration variable was positively correlated: the alfalfa edge density. No local characteristics or landscape structures were associated with the abundance of adult weevils. The abundance of Bathyplectes spp. adults was positively associated to local factors such as the densities of alfalfa weevils and aphids. Few landscape structure variables, such as alfalfa edge density and Simpson’s Diversity Index, had explanatory value only at 250 m buffer radius. The rate of larval parasitism was affected by local variables, such as alfalfa weevil abundance and field age.
Conclusion
Our results provide, for the first time in the Mediterranean region and Europe, evidence of the relative importance of landscape structure and local factors on the abundance of the alfalfa weevil and its larval parasitoids, Bathyplectes spp. The strongest influences were based on local characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alfalfa (Medicago sativa L.) is one of the most economically important forage crops worldwide (Michaud et al. 1988; Frame et al. 1998). In Spain, it covers an area of 250,000 ha, accounting for ~ 20% of European production. In the Ebro Basin (Fig. 1a and b), alfalfa is a common crop in irrigated areas, representing ~ 60% of Spanish production (Delgado and Lloveras 2020).
The alfalfa weevil Hypera postica Gyllenhal (Coleoptera: Curculionidae) is a highly destructive pest specific to alfalfa (Goosey 2012; Saeidi and Moharramipour 2017; Pons and Nuñez 2020). It is native to Eurasia but has a global distribution (Hoffmann 1963). Both adult weevils and larvae feed on alfalfa leaves, but the larvae cause most of the damage, leading to economic losses by impeding plant growth and reducing biomass accumulation (Berberet and McNew1986; Alfaro 2005). In Spain, the alfalfa weevil inflicts most damage during the initial alfalfa cutting in spring (March–April), although they occasionally have an impact on the second cutting too (Pons and Núñez 2020). One or two complete generations may occur, depending on the temperature (Levi-Mourao et al. 2022c). Female weevils begin laying eggs in October after a period of summer estivation, depositing clusters of eggs within alfalfa stems (Domínguez 1989; Alfaro 2005; Pons and Nuñez 2020; Levi-Mourao et al. 2021, 2022b). The resulting larvae hatch between the end of winter and the beginning of spring, and feed on leaves and new plant buds. At the end of the fourth instar, the larvae pupate between the litter and the plant leaflets in white cocoons (Levi-Mourao 2022c).
As a perennial crop and one of the traditional components of arable crop rotations in Spain, alfalfa provides a favorable habitat for many natural enemies of the alfalfa weevil (such as predators and parasitoids) and is much more stable (it remains 3–6 years in the field) than other extensive field crops such as cereals, sunflower or ryegrass (Summers 1998; Núñez 2002; Pellissier et al. 2017; Rand 2017). This natural enemy complex helps to minimize primary and secondary pest outbreaks in alfalfa and its surrounding crops (Summers 1998; Madeira et al. 2019, 2021). The most important natural enemies of alfalfa weevil larvae are solitary larval endoparasitoid wasps of the genus Bathyplectes (Hymenoptera: Ichneumonidae) (Hussain 1975; Flanders et al. 1994; Radcliffe and Flanders 1998; Pellissier et al. 2017). Although Ribes (2012) reported the presence of eight Bathyplectes species in the Iberian Peninsula, only two are known to be associated with the alfalfa weevil: B. anura and B. curculionis (Thomson) (Pons and Nuñez 2020; Levi-Mourao et al. 2022a).
Pest outbreaks and pest control by natural enemies depend not only on local field conditions but also on landscape patterns (With et al. 2002). Some of the literature on the effects of landscape complexity on insect abundance is related to the natural enemies of insect pests in the context of pest control (Symondson et al. 2003; Bianchi et al. 2006; Tscharntke et al. 2012; Rusch et al. 2016). Most insects need to move across the landscape to search for resources (e.g., change of host, feeding or mating), and scaling up from the field to the landscape therefore appears necessary to understand pest control services in crops (Landis et al. 2003; Rand et al. 2006; Tscharntke et al. 2007, 2012; Bianchi et al. 2013). Agricultural landscapes are more dynamic than other landscape types (Petit 2009) because most crops are frequently disrupted by agronomic practices, changes in crop phenology, harvest and/or crop rotations, making them periodically unsuitable to support pests. The impact of landscape patterns on pest abundance and pest control by natural enemies is thus expected to change during the year and between years (Menalled et al. 2003; Bianchi et al. 2006; Rand 2013). Moreover, landscape composition has inconsistent effects on pests and natural enemies, especially when different landscapes are considered (Karp et al. 2018). Studies considering the effect of landscape structure should therefore cover multiple years and should consider the different characteristics of the area, such as crop cover changes among seasons.
When considering a specialist pest and the specific crop it attacks, a positive correlation would be anticipated between pest abundance and the crop area at the landscape scale (Rand et al. 2014). However, this relationship may be nonexistent or even negative in certain cases. For example, some pests require alternative habitats and may live elsewhere during an important part of their life cycle (Östman et al. 2001; Thies et al. 2005, 2008). Furthermore, the abundance of some pests may depend on the presence of non-disturbed elements in the landscape, such as non-treated areas, margins, and woody areas (Ricci et al. 2009). Similarly, a positive correlation would be anticipated between the abundance of specialist natural enemies in the landscape and a larger area of the host crop. However, natural enemies may use alternative food resources or refuges beyond the area covered by the crop. For example, Bathyplectes spp. use alternative food sources such as aphid honeydew and flower nectar, which could have a significant impact on their parasitism levels at both the field and landscape scales (Jacob and Evans 1998, 2000; Evans 2018; Rand and Lundgren 2019).
Pests and their control strategies in alfalfa have mainly been studied at the field scale in Spain. Recognizing the need for larger scales, some studies in the Ebro Basin have reported the relationships between landscape characteristics and insect abundance in crops such as maize and alfalfa (Madeira et al. 2014, 2021; di Lascio et al. 2016; Clemente-Orta et al. 2020). However, none of these studies focused on the alfalfa weevil and its natural enemies. Little is known about the motility of this pest, and how its abundance may be affected by other alfalfa fields or by the surrounding landscape structure. Recently, a parallel study using a different approach but with a similar goal was conducted in the USA to evaluate the effects of landscape structure and local factors on the abundance of the alfalfa weevil and B. curculionis (Pellissier et al. 2022). However, there are significant differences in agricultural practices and landscape structure between the Northern Great Plains of the USA and the Ebro Basin, with the latter being much more heterogeneous and composed of many more small fields per unit of area. Additionally, alfalfa weevil populations can diverge significantly across different geographical areas, including USA and Europe (Sanaei et al. 2019), which could result in different adaptations to local and landscape factors.
We investigated the influence of the surrounding landscape structure (composition and configuration) on the abundance of alfalfa weevil and the abundance and parasitism rates of Bathyplectes spp. We tested five specific hypotheses. First, following the resource concentration hypothesis (Root 1973), the abundance of alfalfa weevil (a specialist pest) should be positively correlated with the abundance of alfalfa in the landscape. Second, the abundance of alfalfa weevil should be positively correlated with the proportion of natural edges (field margins) in the landscape because natural edges facilitate dispersal to new fields and serve as overwintering and estivation areas (Manglitz 1958; Dennis and Fry 1992; Holland and Fahrig 2000; Prokopy et al. 1965, 1967). Third, the abundance of alfalfa weevil larvae (a low mobility insect stage) should be influenced more by local field characteristics and crop management than the surrounding landscape patterns (Blodgett et al. 2000; Goosey et al. 2004; Rand 2013). Fourth, the abundance of Bathyplectes spp. and the parasitism rate should be related to the surrounding landscape characteristics rather than local factors of the alfalfa field, due to the dispersal capacity of these parasitoids in the search for food (Jacob and Evans 1998, 2000; Evans 2018; Rand and Lundgren 2019). Finally, parasitism rates by Bathyplectes spp. should decrease with the higher abundance of alfalfa weevil larvae as previously reported in North America (Rand 2013).
Materials and Methods
Study area
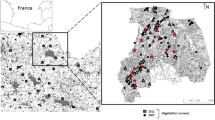
The study was performed in the Ebro Basin (Fig. 1a), at the northeast of the Iberian Peninsula, western Mediterranean area (Fig. 1b). The mean temperature ranges from 1 °C in winter to 30 °C in summer. Annual rainfall is variable, ranging from 200 to 800 mm, and is mainly concentrated in spring and autumn. The mean altitude is 200 m above sea level (asl). In this area, agricultural landscapes are traditionally dominated by arable crops that are managed by the rotation of winter cereals, such as wheat and barley, summer cereals, mainly maize, and alfalfa. Winter cereals are grown from October to June and maize is grown from the end of March/April to October, although the current tendency in non-rotation crop systems is to sow it earlier, during March (Cantero-Martínez et al. 2006; Albajes et al. 2022). Non-cultivated areas, such as forests and woody areas, older fallows, natural habitats, field margins, roads, and irrigation canals are interspersed in these agricultural landscape mosaics. Forests are typically dominated by replanted Pinus halepensis (Mill.) and Mediterranean bushes.
We selected 65 commercial alfalfa fields located in four different counties of the region (Urgell, Segrià, Cinca Medio and Monegros) (Fig. 1b) during 2018, 2019 and 2020 (field characteristics and geographic locations are summarized in Table S1). The fields in each county were separated by at least 2 km within years to avoid potential spatial autocorrelation. The landscape studies were selected along a gradient of high to low alfalfa dominance (Table S2). The selected alfalfa fields were 2, 3 or 4 years old, and were sown with the Aragon variety, derived from the ecotype Aragon, which has been cultivated in the Ebro Valley for decades. As well as tolerating temperatures below –15 °C, it has a short dormancy period, fast development in spring, and regrows after cutting, allowing 5–6 cuts per season under irrigation (Delgado 2020; Delgado and Lloveras 2020). No insect resistance traits are known for this variety. The field sizes ranged from 1 to 7 ha, a common range in this area, and were irrigated by sprinkling or flooding. No pesticides were applied during the study period. The parasitism rate was estimated in 35 fields selected from the original 65, representing the 2019 and 2020 campaigns.
Data records
For each selected alfalfa field, we recorded crop management and field characteristics. We also recorded the landscape coverages at buffer radii of 250, 500 and 1000 m (Fig. 2) from the center of each sampled field, based on our practical constraints in terms of work and time, and following previous studies (Clemente-Orta et al. 2020; Madeira et al. 2021). Each field was sampled for insects once during winter (eggs) and twice (larva and adult) during the first alfalfa intercut, between the beginning of vegetative growth and the first cutting (Pons et al. 2005) in March and April. When possible, sampling was repeated in the same fields in all 3 years of the study (n = 3), but due to crop rotations some fields were sampled only twice (n = 41) or only once (n = 21).
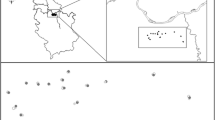
Examples of 1000 m cover type descriptions at the three buffers with low, medium and high alfalfa gradients (a, b, and c respectively). Dark green shows alfalfa patches (AL). Different colors indicate the different cover types initially defined in the landscape (AC: arable crops; AG: water areas; ED: Buildings and urban areas; FO: forest; NC: non-crop; OR: orchards; and VI: roads)
Local variables
Local variables included the perimeter, area, alfalfa age, and irrigation system (sprinkling or flooding) in each field. In the analysis of Bathyplectes spp. (adult abundance and parasitism rate), we included the abundance of alfalfa weevil larvae and aphids as explanatory variables. The perimeter and the area of each selected alfalfa field were calculated using ArcGIS 10.3.3 (Environmental Research Institute—ESRI, Redlands, CA, USA 2015).
Landscape variables
Landscape variables were selected based on previous work in the region (Clemente-Orta et al. 2020a, 2020b; Madeira et al. 2021), and their potential effect on the populations of alfalfa weevil and its larval parasitoid, Bathyplectes spp. ArcGIS software was used to quantify land cover types. Landscape composition was determined by direct field inspections during the first alfalfa cutting (March–April) each year; by using orthophotos from the Plan Nacional de Ortografía Aérea (PNOA) and other reference geographical information maps of the Instituto Geográfico Nacional de España (https://www.ign.es); and by consulting Declaració Agrària (DUN) from the Departament d’Acció Climàtica, Alimentació i Agenda Rural (http://agricultura.gencat.cat/ declaracio-unica-agraria/). The DUN is an annual declaration that must be submitted by the person in charge of the holding, defining the crop and its cultivated area. Thirteen cover types were categorized at the 250 and 500 m buffer radii (Table S2). However, only eight cover types were considered at the 1000 m radius due to the large number of fields within this radius, making it impossible to visit all of them and distinguish between some cover types with the geographical information available (Table S2). Fruit orchards were grouped into one category. They were initially recorded by species due to the higher variability between sampled areas, but the most prevalent ones belonged to the same family (Rosaceae) and in any case they represented a low proportion of the Monegros and Cinca Medio landscapes. Cover types representing a negligible proportion of the landscape (mean < 2%) were not included in the analysis (Table S2). Cover types with similar functions in our system, for example apple and peach orchards, were grouped together (Table S2). For data analyses, the initial cover types were combined into six variables at 250 m and 500 m buffers and five variables at the 1000 m buffer (Table 1).
Landscape configuration was characterized using FRAGSTAT v.14.2 (McGarigal et al. 1995). We selected configuration metrics that capture different information about landscape structure (Hann et al. 2019; McGarigal et al. 1995) and were relevant to the focal species studied: alfalfa edge density, total edge density, patch-Euclidean nearest neighbor, alfalfa-Euclidean nearest neighbor and Simpson’s diversity Index (Table 1).
Insect sampling
The abundance of alfalfa weevil eggs was determined on samples of 200 similar length (30–40 cm) alfalfa stems per field (from the middle of December to the second fortnight of January). Stems were cut using scissors and stored at 5 °C until dissection (within 7 days). The abundance of weevil larvae, weevil adults, Bathyplectes adults and aphids was determined by conducting 180° sweeps with a net 38 cm in diameter. Bathyplectes adults were distinguished from other parasitoids following Ribes’ (2012) identification key. Fields were sampled on two different dates during the first intercut period (from mid-March to the end of April). Twelve samples (five sweeps per sample) were taken in each field on each date. Each field was divided into four sectors, and three samples per sector were collected following the central part of one of the main diagonals. Samples were stored at –20 °C.
On the same sampling dates, we collected an additional sample consisting of 20 sweeps per field, along the main diagonal of the field, to the estimate of larval parasitism in 35 of the selected fields (14 from 2019 and 21 from 2020). Alfalfa weevil larvae were kept in 500-ml polyethylene rearing cages (maximum 50 larvae/cage), covered with mesh to facilitate aeration. Fresh alfalfa was provided every day. Larvae were maintained in a climatic chamber at 22 °C, with a 8:16 (L:D) photoperiod and 50% relative humidity until pupation. Parasitoid puparia were used for morphological identification (Levi-Mourao et al. 2022a).
Data analysis
Spearman rank correlations (Dormann et al. 2013) were used to assess the correlation between landscape structure and field variables (Table S3), to prevent multicollinearity in the statistical models (Legendre 1993; Wagner and Fortin 2005). When strong or very strong correlations between variables were found (Spearman’s rho > 0.59) (Campbell and Swinscow 2009), variables with lower biological relevance were excluded from the models. Moderately correlated variables (Spearman’s rho 0.4–0.59) (Campbell and Swinscow 2009), were not excluded to build the models (Schmidt et al. 2019). For the dependent variables, spatial autocorrelation among fields was tested based on mean values for the 3 year study period, using Moran’s I statistic (Paradis 2019). No significant autocorrelations were detected (Table S4).
The effects of landscape structure and local variables on the abundance of alfalfa weevil (eggs, larvae and adults) and Bathyplectes spp. adults were evaluated by sampling the abundances in two samples collected during the first alfalfa cutting and presenting the average value. For the adult weevils, one outlier (nine times higher than any other value observed, and caused spatial autocorrelation problems) was discarded from the dataset. The data were not distributed normally even after transformation, so gamma-family generalized linear mixed models (GLMMs) were applied because they fitted well the distribution pattern of our data. For the analysis of Bathyplectes spp. parasitism rate, binomial-family GLMM models were used in which we included the weight of the variable (initial number of reared H. postica larvae for parasitism rate estimation). Field and year were always included as random factors. Models were fitted for each spatial scale (250, 500 and 1000 m) using the glmer() function of the lme4 package (Bates et al. 2015). Landscape and local metrics for each model were standardized (mean centered and scaled) before analysis using the caret package (Kuhn 2022).
A multi-model inference approach was used to obtain robust parameter estimates. The dredge() function of the MuMin package (Bartoń 2020) was used to fit all possible combination models, describe the effects of independent variables on each dependent variable, and calculate their associated Akaike information criterion corrected for small sample sizes (AICc). We created an arranged list of models based on the comparison of AICc with the full model values. Model averaging was applied to the model set with ΔAiCc < 2 (Burnham and Anderson 2004) using the model.avg() function from the MuMIn package. To avoid multicollinearity, variance inflation factors (VIFs) for the covariates in the averaged models were calculated using the vif() function from the car package (Fox and Weisberg 2019). Covariates with VIF > 5 were discarded and the analysis was repeated. Lastly, the effects package (Fox et al. 2016) was used to represent the effects in partial residual plots. The function sigtest() from the influence.ME package (Nieuwenhuis et al. 2012) was used to identify influential data points but none were detected. Statistical analyses were conducted using R v4.0.3 (R Development Core Team 2022).
Results
Insect abundance and parasitism rates
We collected 25,024 H. postica eggs, 171,808 larvae and 1,952 adults in the 65 sampled alfalfa fields during the 2018, 2019, and 2020 seasons. We also collected 863 adult Bathyplectes spp. Yearly parasitism rates per field ranged from < 2% to > 30%, with mean values of 15.4% in 2019 and 5.1% in 2020.
Effects of local variables
The abundance of H. postica eggs was highest at all scales when the field was sprinkler irrigated (Fig. 3a–c). Likewise, the abundance of H. postica larvae increased with sprinkler irrigation at the 500-m and 1000-m scales (Fig. 3e, f). The field perimeter had a positive association with the abundance of H. postica eggs at the 1000-m scale (Fig. 3d) and larvae, at all scales (Fig. 3g–i). In contrast, local variables had no significant effect on the abundance of H. postica adults.
The abundance of Bathyplectes spp. adults was positively associated with the abundance of alfalfa weevil larvae at all scales (Fig. 4a–c) and so was the parasitism rate (Fig. 4j–l). Furthermore, the abundance of parasitoid adults was positively associated with the abundance of aphids (Fig. 4d–f). Age was found to be a significant factor affecting parasitism rates, with lower rates observed in older fields (Fig. 4g–i). Table 2 shows the significant effects of alfalfa field variables on H. postica and Bathyplectes spp. (adults and parasitism rate). The total effects of all local variables are shown in Table S5.
Partial residual plots showing the significant local variables (H. postica larvae, aphids and age) influencing the abundance of Bathyplectes spp. adults (a–f) and parasitism rate (g–l). From figures a–f, the Y-axes are on the log-scale, and from figures g–l, they are on the logit-scale to preserve the linear structure of the model. The blue shaded areas represent 95% confidence intervals
Effects of landscape variables
The landscape structure had a relatively weak effect on the abundance of H. postica and Bathyplectes spp. (adult abundance and parasitism rate). None of the composition variables had a significant effect. Only one configuration metric (alfalfa edge density) was positively associated with the abundance of H. postica larvae across all landscape scales (Fig. 5a–c). Conversely, the landscape structure had no significant effects on H. postica adults and eggs. The most parsimonious models are shown in Table S5, and the significant effects of landscape variables on H. postica (abundance) and Bathyplectes spp. (adult abundance and parasitism rate) are shown in Table 2.
At the 250-m scale, there was a negative relationship between the abundance of Bathyplectes spp. adults and Simpson’s diversity index (Fig. 6a) and a positive correlation with alfalfa edge density (Fig. 6b). However, no effects were observed at the 500 m and 1000 m scales, and the landscape structure also had no impact on parasitism rates.
Partial residual plots showing the significant landscape variables (Simpson’s diversity index and alfalfa edge density) and their effect on the abundance of Bathyplectes spp. adults. Y-axes are on the log-scale to preserve the linear structure of the model. Blue shaded areas represent 95% confidence intervals
Discussion
Over the last two decades, several studies in the Ebro Basin have described the composition, ecological role and abundance of insect populations that live in alfalfa, which is a prevalent forage crop in the region. These studies concluded that alfalfa is an important reservoir of natural enemies that provides biocontrol services to alfalfa and other neighboring crops (Núñez 2002; Pons et al. 2005; Madeira et al. 2014, 2019; Di Lascio et al. 2016; Batuecas et al. 2022). However, these studies did not consider the effect of landscape structure or field characteristics on major pests such as the alfalfa weevil, which causes extensive damage at the larval stage in most areas where alfalfa is cultivated (Goosey 2012; Saeidi and Moharramipour 2017; Soroka et al. 2019; Levi-Mourao et al. 2022b). Indeed, little is known about the factors that regulate the abundance of this pest in Europe. Here, we show for the first time the effects of field characteristics and landscape structure on the alfalfa weevil and its larval parasitoids in Mediterranean alfalfa crops.
Contrary to our first hypothesis, we observed no association between the abundance of alfalfa weevil and the proportion of alfalfa in the landscape. The abundance of specialized herbivores tends to correlate with the extent of their host crop, typically being more numerous in simple environments characterized by low plant diversity (O’Rouke et al. 2011; Root 1973). Consistent with this expectation, the abundance of alfalfa weevil in North America was recently shown to correlate with the proportion of alfalfa in the landscape, particularly at scales ≥ 2000 m (Pellissier et al. 2022). Our contrasting results may be due to the greater heterogeneity of the agricultural landscape in the Ebro Valley, which consists of smaller fields (Clemente-Orta et al. 2020; Madeira et al. 2021) compared to the more uniform landscape of larger fields in North America (Pellissier et al. 2022). Dispersal mortality and fitness costs become significant factors when specialist herbivores are compelled to relocate across greater diversity of habitats in pursuit of their host crop (Chaplin-Kramer et al. 2011). Altogether, these results illustrate how the availability and distribution of resources can influence pest dynamics in different agricultural landscapes (O'Rourke and Peterson 2017; Fahrig and Jonsen 1998; Samaranayake and Costamagna 2018). In our case, we hypothesize that alfalfa weevil adult populations that must cross fields of other crops or orchards treated with insecticides (Clemente-Orta et al. 2020; Madeira et al. 2021) to reach the nearest alfalfa fields may suffer high mortality during dispersal.
According to our second and third hypotheses, alfalfa weevil populations are associated with the occurrence of natural edges in the landscape, and larval abundance in the field mostly depends on the field characteristics. We found that larval abundance was associated with greater alfalfa edge densities and longer field perimeters, the latter showing also a positive correlation with the number of H. postica eggs at 1000 m. In this context, both variables may be considered as a proxy of the margins surrounding an alfalfa field, which act as natural refugees for alfalfa herbivores including the alfalfa weevil, supporting their population growth (Landis et al. 2003; Madeira et al. 2021; Werling and Gratton 2008). Several studies have reported that adult weevils aestivating in the summer become concentrated along the borders of alfalfa fields, allowing rapid re-infestation for feeding and reproduction in the autumn (Manglitz 1958; Prokopy and Gyrisco 1965; Prokopy et al. 1967; Saeidi and Moharramipour 2017; Pellissier et al 2022). This re-infestation by adults is indirectly supported by the abundance of eggs and larvae recorded in winter and spring, respectively. Alfalfa was not irrigated during the time of our sampling, but the irrigation system also influenced the abundance of H. postica eggs and larvae, with higher numbers of both stages found in fields irrigated by sprinkling rather than flooding. This difference may be caused by the absence of cutting along the route of the sprinkler supply pipe, ensuring that some plants effectively function as field refuges, continuously available for oviposition (Levi-Mourao et al. 2022b).
None of the local or landscape structure factors influenced the abundance of H. postica adults. However, this may not be relevant to the generation of adults arising from the spring larvae collected in our samples, which are expected to stay in the field for aestivation during the summer (Prokopy and Gyrisco 1965; Prokopy et al. 1967; Manglitz 1958; Levi-Mourao et al. 2022c). Instead, landscape structure and field characteristics may affect adults recovering after aestivation because they are responsible for mating, oviposition and field colonization during the autumn months (Manglitz 1958; Prokopy and Gyrisco 1965; Prokopy et al. 1967; Levi-Mourao et al. 2022c).
In contrast to our fourth hypothesis, landscape structure variables had only a limited explanatory value concerning the abundance of Bathyplectes spp. adults and none concerning the rate of parasitism. Instead, our results indicated that local variables have a greater influence on both outcomes. However, the abundance of Bathyplectes spp. adults correlated with the abundance of aphids and alfalfa weevil larvae, which probably reflects the resources available in the fields that support parasitoids: weevil larvae hosts and aphid honeydew (Tscharntke et al. 2016; Pellissier et al. 2022). Several studies have shown that the presence of aphids in the field can benefit Bathyplectes spp. by providing food (honeydew) thus increasing parasitoid longevity and their control over the alfalfa weevil larval population (Jacob and Evans 1998; Rand and Lundgren 2019). However, we are the first to demonstrate that aphid abundance favors Bathyplectes spp. adults on a local scale.
The abundance of Bathyplectes spp. adults also correlated positively with alfalfa edge density and negatively with Simpson’s diversity index, but only at the 250-m scale. The positive association with alfalfa edge density may result from the search for food resources near the field borders, where several nectar sources from wild flowers are likely to be found. Most of these spring weeds belong to the family Asteraceae, including the common dandelion (Taraxacum officinale Weber), and have been recognized in other studies for their ability to enhance the longevity and reproductive performance of Bathyplectes spp. (Maingay et al. 1991; Jervis et al. 1993; Jacob and Evans 2000). This result is consistent with the negative association with Simpson’s diversity index and it can be assumed since higher alfalfa edge density corresponds to a larger percentage of the area being occupied by alfalfa crop, indicating a reduction in the surface area of other crops.
We found that the Bathyplectes spp. parasitism rate was negatively correlated with alfalfa age. Given that alfalfa is a pluriannual crop, the density of weeds increases as the crop age and are overexploited (Taberner 2020; Fahrig and Jonsen 1998; Zumoffen et al. 2012), thus reducing the availability of host plants for weevil larvae. Since we did not quantify weed density in our focal fields, further research is needed to determine the role of weeds in the system. In our study the parasitism rate was positively associated with the abundance of weevil larvae, contradicting earlier reports of a negative association (Rand 2013; Pellissier et al. 2022). In these two previous studies, spanning one or two seasons, this negative association was attributed to the inability of B. curculionis to keep up with heavy alfalfa weevil infestations. However, the host–parasitoid relationship can change between years, depending on the population dynamics (Al Ayedh et al. 1996; Costamagna et al. 2004). The differences between our results and the earlier studies (Rand 2013; Pellissier et al. 2022) may also be due to the different proportions of Bathyplectes species that we found. Both previous studies only considered B. curculionis, the major parasitoid of alfalfa weevil larvae in many regions of North America (Rand 2013; Berberet and Bisges 1998; Radcliff and Flanders 1998). In contrast, B. anura accounts for 90% of Bathyplectes specimens collected in the Ebro Basin (Pons and Núñez 2020; Levi-Mourao et al. 2022b). B. anura also has a higher reproductive capacity than B. curculionis, is faster at searching and host-handling, and globally has a more aggressive behavior (Harcourt 1990; Levi-Mourao et al. 2022b).
Conclusion
In conclusion, pests, natural enemies and biological control services do not always respond to the complexity of landscape structure (Rusch et al. 2016; Tscharntke et al. 2016; Karp et al. 2018). Our 3-year study of H. postica at three development stages, and its larval parasitoid Bathyplectes spp., provides the first evidence that H. postica and Bathyplectes spp. respond more to local factors than landscape characteristics under Mediterranean agricultural conditions in Europe. The abundance of alfalfa weevil eggs and larvae was positively associated with longer field perimeters and a sprinkling irrigation system, whereas the landscape composition had no effect and only one configuration metric (alfalfa edge density) explained the larval abundance. The abundance of Bathyplectes spp. correlated with the abundance of alfalfa weevil larvae and aphids, highlighting the role of aphids as a driver, probably by providing honeydew as food. Finally, Bathyplectes spp. parasitism rates mainly depended on the age of the alfalfa plants and the abundance of alfalfa weevil larvae.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Al Ayedh HY, Kondratieff BC, Blodgett SL, Peairs FB (1996) Evaluation of hymenopterous biological control agents of the alfalfa weevil larvae Hypera postica (Coleoptera: Curculionidae) in Northcentral Colorado. J Kans Entomol 69:326–336
Albajes R, Pons X, Cantero-Martínez C, Achón MA, López C, Eizaguirre M (2022) Intensificación de las rotaciones de cereales en la Cuenca del Ebro: efecto en la incidencia de plagas y virosis. Phytoma España 335:36–42
Alfaro A (2005) Entomología Agraria. Los parásitos animales de las plantas cultivadas. Publicaciones de la Excma, Diputación de Soria
Bartoń K (2020) MuMIn: title multi-model inference. R package version: 1.43.6. MuMIn.pdf (r-project.org)
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using Lme4. J Stat Softw 67:1–48
Batuecas I, Alomar O, Castañé C, Gallardo-Montoya L, Agustí N (2022). Disentangling arthropod and plant resources consumed by Orius spp. in peach and alfalfa crops by metagenomic analysis. https://doi.org/10.21203/rs.3.rs-1474466/v1
Berberet RC, Bisges AD (1998) Potential for competition among natural enemies of larvae of Hypera postica (Coleoptera: Curculionidae) in the southern plains. Environ Entomol 27:743–751
Berberet RC, McNew RW (1986) Reduction in yield and quality of leaf and stem components of alfalfa forage due to damage by larvae of Hypera postica (Coleoptera: Curculionidae). J Econ Entomol 79:212–218
Bianchi FJJA, Booij CJH, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc r Soc B Biol Sci 273:1715–1727
Bianchi FJJA, Schellhorn NA, Cunningham SA (2013) Habitat functionality for the ecosystem service of pest control: reproduction and feeding sites of pests and natural enemies. Agric for Entomol 15:12–23
Blodgett SL, Lenssen AW, Cash SD (2000) Harvest with ranking for control of alfalfa weevil (Coleoptera: Curculionidae). J Entomol Sci 35:129–135
Burnham KP, Anderson DR (2004) Multimodel Inference: Understanding AIC and BIC in Model Selection. Soc Methods Res 33:261–304
Campbell MJ, Swinscow TDV (2009) Statistics at square one, 11th edn. Wiley-Blackewll, Chichester
Cantero-Martínez C, Santiveri P, Lloveras J, Chocarro C (2006) Agronomy of field crops, in: Estany, J. (Ed.), Agriculture and Agri-Food Production in Perspective. Profile of the Sector in Catalonia. Universitat de Lleida, Lleida, Spain.
Chaplin-Kramer R, O’Rourke ME, Blitzer EJ, Kremen C (2011) A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol Lett 14:922–932
Clemente-Orta G, Madeira F, Batuecas I, Sossai S, Juárez-Escario A, Albajes R (2020) Changes in landscape composition influence the abundance of insects on maize: The role of fruit orchards and alfalfa crops. Agric Ecosyst Environ 291:106805
Costamagna AC, Menalled FD, Landis DA (2004) Host density influences parasitism of the armyworm Pseudaletia unipuncta in agricultural landscapes. Basic Appl Ecol 5:347–355
Delgado I (2020) Botánica. In: Lloveras J, Delgado I, Chocarro C (eds) La Alfalfa - Agronomia y Utilización-. Edicions de la Universitat de Lleida. Centro de Investigación y Tecnología Agroalimentaria de Aragón, Lleida- Zaragoza, Spain, pp 33–42
Delgado I, Lloveras J (2020) Historia y distribución de la alfalfa. In: Lloveras J, Delgado I, Chocarro C (eds) La Alfalfa, Agronomía y Utilización. Edicions de la Universitat de Lleida. Centro de Investigación y Tecnología Agroalimentaria de Aragón, Lleida-Zaragoza Spain, pp 17–32
Dennis P, Fry GLA (1992) Field margins: can they enhance natural enemy population densities and general arthropod diversity on farmland? Agric Ecosyst Environ 40:95–115
di Lascio A, Madeira F, Costantini ML, Rossi L, Pons X (2016) Movement of three aphidophagous ladybird species between alfalfa and maize revealed by carbon and nitrogen stable isotope analysis. Biocontrol 61:35–46
Domínguez F (1989) Plagas y enfermedades de las plantas cultivadas, 8th ed. Mundi Prensa, Madrid, Spain.
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, Mcclean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography (cop) 36:27–46
ESRI (2015). Arcgis Desktop Version 10.3.1. Environmental Systems Research Institute, Redlands, California, USA.
Evans EW (2018) Dispersal in host-parasitoid interactions: crop colonization by pests and specialist enemies. InSects 9:134
Fahrig L, Jonsen I (1998) Effect of habitat patch characteristics on abundance and diversity of insects in an agricultural landscape. Ecosystems 1:197–205
Flanders KL, Radcliffe EB, Krueger CA (1994) Natural enemies of alfalfa weevil, Hypera postica (Coleoptera: Curculionidae), in Minnesota. Gt Lakes Entomol 27:7–18
Fox J, Weisberg S (2019) An {R} Companion to Applied Regression, Third Edition. Thousand Oaks CA: Sage. URL: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Fox J, Weisberg S, Price B, Friendly M, Hong J, Anderson R, Firth D, Taylor S (2016) Effects: effect displays for linear, generalized linear, and other models. R Package Version: 4.2–0. https://cran.r-project.org/web/packages/effects/effects.pdf
Frame J, Charlton J, Laidlaw A (1998) Temperate forrage legumes. CAB international. London, UK.
Goosey HB (2012) A degree-day model of sheep grazing influence on alfalfa weevil and crop characteristics. J Econ Entomol 105:102–112
Goosey HB, Harfield PJ, Blodgett SL, Cash SD (2004) Evaluation of alfalfa weevil (Coleoptera: Curculionidae) densities and regrowth characteristics of alfalfa grazed by sheep in winter and spring. J Entomol Sci 35:598–610
Hann NL, Zhang Y, Landis DA (2019) Predicting landscape configuration effects on agricultural pest supression. Trends Ecol Evol 35:175–186
Harcourt DG (1990) Displacement of Bathyplectes curculionis (Thoms.) (Hymenoptera: Ichneumonidae) by B. anurus (Thoms.) in eastern Ontario populations of the alfalfa weevil, Hypera postica (Gyll.) (Coleoptera: Curculionidae). Can Entomol 122:641–645
Hoffmann A (1963) Sous-famille des Curculionidae, Tribu des Hyperini, Les Hypera (syn: Phytonomus), in: Balachowsky, A.S. (Ed.), Entomologie Appliquée a l’agriculture. Tome I. Coléoptères. Second Volume. Masson et Cíe., Paris, Francia, pp. 984–989.
Holland J, Fahrig L (2000) Effect of woody borders on insect density and diversity in crop fields: a landscape-scale analysis. Agric Ecosyst Environ 78:115–122
Hussain M (1975) Predators of the alfalfa weevil, Hypera postica in Western Nevada: a greenhouse study. (Coleoptera: Curculionidae). J New York Entomol Soc 83:226–228
Jacob HS, Evans EW (1998) Effects of sugar spray and aphid honeydew on field populations of the parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ Entomol 27:1563–1568
Jacob HS, Evans EW (2000) Influence of carbohydrate foods and mating on longevity of the parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ Entomol 29:1088–1095
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 27:67–105
Karp DS, Chaplin-Kramer R, Meehan TD, Martin EA, DeClerck F, Grab H, Gratton C, Hunt L, Larsen AE, Martínez-Salinas A, O’Rourke ME, Rusch A, Poveda K, Jonsson M, Rosenheim JA, Schellhorn NA, Tscharntke T, Wratten SD, Zhang W, Iverson AL, Adler LS, Albrecht M, Alignier A, Angelella GM, Anjum MZ, Avelino J, Batáry P, Baveco JM, Bianchi FJJA, Birkhofer K, Bohnenblust EW, Bommarco R, Brewer MJ, Caballero-López B, Carrière Y, Carvalheiro LG, Cayuela L, Centrella M, Ćetković A, Henri DC, Chabert A, Costamagna AC, De la Mora A, de Kraker J, Desneux N, Diehl E, Diekötter T, Dormann CF, Eckberg JO, Entling MH, Fiedler D, Franck P, van Veen FJF, Frank T, Gagic V, Garratt MPD, Getachew A, Gonthier DJ, Goodell PB, Graziosi I, Groves RL, Gurr GM, Hajian-Forooshani Z, Heimpel GE, Herrmann JD, Huseth AS, Inclán DJ, Ingrao AJ, Iv P, Jacot K, Johnson GA, Jones L, Kaiser M, Kaser JM, Keasar T, Kim TN, Kishinevsky M, Landis DA, Lavandero B, Lavigne C, Le Ralec A, Lemessa D, Letourneau DK, Liere H, Lu Y, Lubin Y, Luttermoser T, Maas B, Mace K, Madeira F, Mader V, Cortesero AM, Marini L, Martinez E, Martinson HM, Menozzi P, Mitchell MGE, Miyashita T, Molina GAR, Molina-Montenegro MA, O’Neal ME, Opatovsky I, Ortiz-Martinez S, Nash M, Östman Ö, Ouin A, Pak D, Paredes D, Parsa S, Parry H, Perez-Alvarez R, Perović DJ, Peterson JA, Petit S, Philpott SM, Plantegenest M, Plećas M, Pluess T, Pons X, Potts SG, Pywell RF, Ragsdale DW, Rand TA, Raymond L, Ricci B, Sargent C, Sarthou JP, Saulais J, Schäckermann J, Schmidt NP, Schneider G, Schüepp C, Sivakoff FS, Smith HG, Whitney KS, Stutz S, Szendrei Z, Takada MB, Taki H, Tamburini G, Thomson LJ, Tricault Y, Tsafack N, Tschumi M, Valantin-Morison M, van Trinh M, van der Werf W, Vierling KT, Werling BP, Wickens JB, Wickens VJ, Woodcock BA, Wyckhuys K, Xiao H, Yasuda M, Yoshioka A, Zou Y (2018) Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc Natl Acad Sci 115:E7863–E7870
Kuhn M (2022) Caret: Classification and Regression Training. R package version 6.0–93, https://CRAN.R-project.org/package=caret.
Landis DA, Wratten SD, Gurr GM (2003) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45:175–201
Legendre P (1993) Spatial autocorrelation: trouble or new paradigm? Ecology 74:1659–1673
Levi-Mourao A, Madeira F, Meseguer R, García A, Pons X (2021) Effects of temperature and relative humidity on the embryonic development of Hypera postica Gyllenhal (Col.: Curculionidae). Insects 12(3):250
Levi-Mourao A, Muñoz P, Cerda-Bennasser P, Meseguer R, Pons X (2022a) Molecular and morphological identification of the alfalfa weevil larval parasitoids Bathyplectes anura and Bathyplectes curculionis to estimate the rate of parasitism. Biocontrol 2022:1–12
Levi-Mourao A, Núñez E, García A, Meseguer R, Pons X (2022b) Alfalfa winter cutting Effectiveness against the alfalfa weevil, Hypera postica (Gyllenhal) (Coleoptera: Curculionidae) and effect on its rate of parasitism due to Bathyplectes spp. (Hymenoptera: Ichneumonidae). Crop Prot 152:105858
Levi-Mourao A, Madeira F, Meseguer R, Pons X (2022c) Effects of temperature on the fitness of the alfalfa weevil (Hypera postica). Pest Manag Sci 78:4223–4233
Madeira F, di Lascio A, Carlino P, Costantini ML, Rossi L, Pons X (2014) Stable carbon and nitrogen isotope signatures to determine predator dispersal between alfalfa and maize. Biol Control 77:66–75
Madeira F, di Lascio A, Costantini ML, Rossi L, Rösch V, Pons X (2019) Intercrop movement of heteropteran predators between alfalfa and maize examined by stable isotope analysis. J Pest Sci 92:757–767
Madeira F, Clemente-Orta G, Alomar O, Batuecas I, Sossai S, Albajes R (2021) Land use alters the abundance of herbivore and predatory insects on crops: the case of alfalfa. J Pest Sci 1:1–19
Maingay HM, Bugg RL, Carlson RW, Davidson NA (1991) Predatory and parasitic wasps (Hymenoptera) feeding at flowers of sweet fennel (Foeniculum vulgare Miller var. dulce Battandier & Trabut, Apiaceae) and spearmint (Mentha spicata L., Lamiaceae) in Massachusetts. Biol Agric Hortic 7:363–383
Manglitz GR (1958) Aestivation of the alfalfa weevil. J Econ Entomol 51:506–508
McGarigal K, Marks B (1995) FRAGSTATS: spatial analysis program for quantifying landscape structure. USDA Forest Service Gen. Tech. Rep. PNW-GTR-351. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR.
Menalled FD, Costamagna AC, Marino PC, Landis DA (2003) Temporal variation in the response of parasitoids to agricultural landscape structure. Agric Ecosyst Environ 96:29–35
Michaud R, Lehman WF, Rumbaugh MD (1988) World distribution and historical development, in: Hanson, A.A., Barnes, D.K., Hill, R.R. (Eds.), Alfalfa and Alfalfa Improvement. Agronomy 29. American Society of America, Agronomy, Crop Science Society of America, Soil Science Society of America, Madison,WI., USA, pp. 25–91.
Nieuwenhuis R, Grotenhuis M, Pelzer B (2012) Influence.ME: tools for detecting influential data in mixed effects models. R J 4:38–47
Núñez E (2002) La alfalfa como reservorio de enemigos naturales. PhD thesis. Universitat de Lleida.
O’Rourke ME, Peterson MJ (2017) Extending the ‘resource concentration hypothesis’ to the landscape-scale by considering the dispersal mortality and fitness costs. Agric Ecosyst Environ 249:1–3
O’Rourke ME, Rienzo-Stack K, Power AG (2011) A multi-scale, landscape approach to predicting insect populations in agroecosystems. Ecol Appl 21:1782–1791
Östman Ö, Ekbom B, Bengtsson J (2001) Landscape heterogeneity and farming practice influence biological control. Basic Appl Ecol 2:365–371
Paradis E (2019) Ape: Analyses of phylogenetics and evolution. R Package Version 5.3. http://cran.r-project.org/web/package/ape/ape.pdf
Pellissier ME, Nelson Z, Jabbour R (2017) Ecology and management of the alfalfa weevil (Coleoptera: Curculionidae) in Western United States alfalfa. J Integr Pest Manag 8:5
Pellissier ME, Rand TA, Murphy MA, Jabour R (2022) Landscape composition and management history affect alfalfa weevil but not its parasitoid. Environ Entomol 54:892–900
Petit S (2009) The dimensions of land use change in rural landscapes: lessons learnt from the GB countryside surveys. J Environ Manage 90:2851–2856
Pons X, Nuñez E (2020) Plagas da la alfalfa: Importancia, daños y estrategias de control. In: Lloveras J, Delgado I, Chocarro C (eds) La Alfalfa, Agronomía y Utilización. Edicions de la Universitat de Lleida, Lleida, Spain, pp 167–202
Pons X, Núñez E, Lumbierres B, Albajes R (2005) Epigeal aphidophagous predators and the role of alfalfa as a reservoir of aphid predators for arable crops. Eur J Entomol. https://doi.org/10.14411/eje.2005.074
Prokopy RJ, Gyrisco GG (1965) Summer migration of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae). Ann Entomol Soc Am 58:630–641
Prokopy RJ, Armbrust EJ, Cothran WR, Gyrisco GG (1967) Migration of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae), to and from estivation sites. Ann Entomol Soc Am 60:26–31
R Development Core Team (2022) R: A language and environment for statistical computing. R Foundation for statistical computing. Vienna, Austria. Available online at http://www.R-project.org/
Radcliffe EB, Flanders KL (1998) Biological control of alfalfa weevil in North America. Integr Pest Manag Rev 3:225–242
Rand TA (2013) Host density drives spatial variation in parasitism of the alfalfa weevil, Hypera postica, across dryland and irrigated alfalfa cropping systems. Environ Entomol 42:116–122
Rand TA (2017) Assessing the role of generalist predators in the biological control of alfalfa weevil (Coleoptera: Curculionidae). Can Entomol 149:525–533
Rand TA, Lundgren JG (2019) Quantifying temporal variation in the benefits of aphid honeydew for biological control of alfalfa weevil (Coleoptera: Curculionidae). Environ Entomol 48:141–146
Rand TA, Tylianakis JM, Tscharntke T (2006) Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol Lett 9:603–614
Rand TA, Waters DK, Blodgett SL, Knodel JJ, Harris MO (2014) Increased area of suitable host crop increases herbivore pressure in intensified agricultural landscapes. Agric Ecosyst Environ 186:135–143
Ribes A (2012) Himenòpters de Ponent. http://ponent.atspace.org/fauna/ins/index.htm (accessed 5/3/21).
Ricci B, Franck P, Toubon JF, Bouvier JC, Sauphanor B, Lavigne C (2009) The influence of landscape on insect pest dynamics: a case study in southeastern France. Landsc Ecol 24:337–349
Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleraceae). Ecol Monogr 43:95–124
Rusch A, Chaplin-Kramer R, Gardiner MM, Hawro V, Holland J, Landis D, Thies C, Tscharntke T, Weisser WW, Winqvist C, Woltz M, Bommarco R (2016) Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric Ecosyst Environ 221:198–204
Saeidi M, Moharramipour S (2017) Physiology of cold hardiness, seasonal fluctuations, and cryoprotectant contents in overwintering adults of Hypera postica (Coleoptera: Curculionidae). Environ Entomol 46:960–966
Samaranayake KGLI, Costamagna A (2018) Levels of predator movement between crop and neighboring habitats explain pest supression in soybean across a gradient of agricultural landscape complexity. Agric Ecosyst Environm 259:135–146
Sanaei E, Husemann M, Seiedy M, Rethwisch M, Tuda M, Toshova TB, Kim MJ, Atanasova D, Kim I (2019) Global genetic diversity, lineage distribution, and Wolbachia infection of the alfalfa weevil Hypera postica (Coleoptera: Curculionidae). Ecol Evol 7:9546–9563
Schmidt JM, Whitehouse TS, Green K, Krehenwinkel H, Schmidt-Jeffris R, Sial AA (2019) Local and landscape-scale heterogeneity shape spotted wing drosophila (Drosophila suzukii) activity and natural enemy abundance: Implications for trophic interactions. Agric Ecosyst Environ 272:86–94
Soroka J, Grenkow L, Cárcamo H, Meers S, Barkley S, Gavloski J (2019) An assessment of degree-day models to predict the phenology of alfalfa weevil (Coleoptera: Curculionidae) on the Canadian Prairies. Can Entomol 152:110–129
Summers CG (1998) Integrated pest management in forage alfalfa. Integr Pest Manag 3:127–154
Symondson WOC, Sunderland KD, Greenstone MH (2003) can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Taberner A (2020) Control de malas hierbas. In: Lloveras J, Delgado I, Chocarro C (eds) La Alfalfa, Agronomía y Utilización. Edicions de la Universitat de Lleida, Centro de Investigación y Tecnología Agroalimentaria de Aragón, Lleida- Zaragoza, Spain, pp 253–272
Thies C, Roschewitz I, Tscharntke T (2005) The landscape context of cereal aphid-parasitoid interactions. Proc r Soc B Biol Sci 272:203–210
Thies C, Steffan-Dewenter I, Tscharntke T (2008) Interannual landscape changes influence plant–herbivore–parasitoid interactions. Agric Ecosyst Environ 125:266–268
Tscharntke T, Bommarco R, Clough Y, Crist TO, Kleijn D, Rand TA, Tylianakis JM, Van Nouhuys S, Vidal S (2007) Conservation biological control and enemy diversity on a landscape scale. Biol Control 43:294–309
Tscharntke T, Tylianakis JM, Rand TA, Didham RK, Fahrig L, Batáry P, Bengtsson J, Clough Y, Crist TO, Dormann CF, Ewers RM, Fründ J, Holt RD, Holzschuh A, Klein AM, Kleijn D, Kremen C, Landis DA, Laurance W, Lindenmayer D, Scherber C, Sodhi N, Steffan-Dewenter I, Thies C, van der Putten WH, Westphal C (2012) Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biol Rev 87:661–685
Tscharntke T, Karp DS, Chaplin-Kramer R, Batáry P, DeClerck F, Gratton C, Hunt L, Ives A, Jonsson M, Larsen A, Martin EA, Martínez-Salinas A, Meehan TD, O’Rourke M, Poveda K, Rosenheim JA, Rusch A, Schellhorn N, Wanger TC, Wratten S, Zhang W (2016) When natural habitat fails to enhance biological pest control – Five hypotheses. Biol Conserv 204:449–458
Wagner HH, Fortin MJ (2005) Spatial analysis of landscapes: concepts and statistics. Ecology 86:1975–1987
Werling BP, Gratton C (2008) Influence of field margins and landscape context on ground beetle diversity in Wisconsin (USA) potato fields. Agric Ecosyst Environ 128:104–108
With KA, Pavuk DM, Worchuck JL, Oates RK, Fisher JL (2002) Threshold effects of landscape structure on biological control in agroecosystems. Ecol Appl 12:52–65
Zumoffen L, Salto C, Salvo A (2012) Preliminary study on parasitism of aphids (Hemiptera: Aphididae) in relation to characteristics of alfalfa fields (Medicago sativa L.) in the Argentine Pampas. Agric Ecosyst Environ 159:49–54
Acknowledgements
We thank Aldahra Europe, Cooperative of Bellvís – Verge de les Sogues, Cooperative of Bell-lloc, Granja San José, Osés-Nafosa Group and Josep Piqué for allowing us to use their commercial alfalfa fields for our study. We also thank Marta Franch and Dra. Addy García for technical support. We would like to thank Dr. Richard M Twyman for English language editing.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This project was funded by the Ministerio de Ciencia e Innovación of the Spanish Government (project AGL2017-84127-R). Alexandre Levi-Mourao was funded by a Jade plus grant from the Universitat de Lleida, and Roberto Meseguer was funded by an FPI grant from the Ministerio de Ciencia e Innovación of the Spanish Government. The authors thank the Portuguese Foundation for Science and Technology (FCT) for the financial support to the Research Centre for Natural Resources, Environment and Society—CERNAS (UIDB/00681/2020; 10.54499/UIDP/00681/2020).
Author information
Authors and Affiliations
Contributions
Conceptualization: Xavier Pons; Data curation: Alexandre Levi-Mourao, Roberto Meseguer, José Martinez-Casasnovas; Formal analysis: Alexandre Levi-Mourao, Roberto Meseguer, Filipe Madeira, Alejandro Costamagna; Funding acquisition and resources: Xavier Pons; Investigation: Alexandre Levi-Mourao, Roberto Meseguer; Methodology and validation: Alexandre Levi-Mourao, Xavier Pons; Supervision: Xavier Pons, Filipe Madeira, José Martinez-Casasnovas, Alejandro Costamagna; Writing—original draft: Alexandre Levi-Mourao, Xavier Pons; Writing—review & editing: Alexandre Levi-Mourao, Roberto Meseguer, Filipe Madeira, José Martinez-Casasnovas, Alejandro Costamagna, Xavier Pons. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Levi-Mourao, A., Meseguer, R., Madeira, F. et al. Local factors have a greater influence on the abundance of alfalfa weevil and its larval parasitoids than landscape complexity in heterogeneous landscapes. Landsc Ecol 39, 143 (2024). https://doi.org/10.1007/s10980-024-01949-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10980-024-01949-2