Abstract
Context
Maintaining a balance between semi-natural habitats and arable land is not always feasible for farmers. The promotion of biological control agents can be addressed through management at farm or field level, and/or by deploying lower intensity, biodiversity-friendly practices which can act either directly or indirectly through their effect of the plant community.
Objectives
We studied the effects on cereal aphids and their parasitoids of agricultural management at field and landscape levels. We tested the effect of organic and conventional farming, and of the within field characteristics, on the cereal aphid-parasitoid community, across a gradient of organic farming aggregation and of percentage of arable land.
Methods
In spring 2015, we sampled aphid populations in 30 cereal fields in five agricultural areas in Catalonia (Spain) with contrasting levels of organic farming aggregation. In each field, we also assessed weed and crop cover. As landscape variables, we calculated the Percentage of Agricultural Land (PAL) and the Percentage of Organically Managed Land (POML) in a 500-m buffer around each field. We sampled cereal tillers 3 m from the field edges and collected all aphids detected. In addition, we reared mummies (parasitized aphids) until they hatched.
Results
Our results show that management at landscape level has significant effects on parasitism rates: a higher proportion of surrounding fields under organic management increased the amount of parasitism, as did less agricultural land cover. On the other hand, aphid populations were mainly affected by two in-field factors, namely, crop density and crop variety. Differences in weed communities did not seem to have any effects on either aphids or parasitoids.
Conclusions
Rather than concentrating on the individual management of fields, a coordinated implementation of organic farming at landscape level would seem to be a much better strategy for improving the biological control of aphids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Farmers often plan agricultural management at field scale in a highly individualistic way. However, many studies have shown that the ecological processes that sustain agriculture operate at higher landscape levels (Thies and Tscharntke 1999; Steffan-Dewenter et al. 2001; Vollhardt et al. 2010; Bianchi et al. 2013; Dainese et al. 2019) and thus management practices could be optimized by implementation at landscape level (González-Díaz et al. 2012), as some models suggest. One of the processes that helps sustain agricultural production is biological control, which involves the interaction of pests and their natural enemies.
Winter cereals, which are extensively sown in Europe (Holland et al. 2017), cover approximately 21.8 million ha and in 2021 produced over 150 million tonnes of cereals (Eurostat 2022). Aphids are the main arthropod pest in these crops (Vickerman and Wratten 1979). Apart from diverting part of the energy that the plants produce, aphids are the major vector of yellow dwarf disease, one of the most important viral diseases in cereal crops worldwide and one that causes yield losses of up to 80% (Duelli and Obrist 2003; Gray and Gildow 2003; Grauby et al. 2022). Aphids live on the tillers of cereal plants, where adults feed and reproduce, and complete their life cycles in fields during the cropping season (Blackman and Eastop 2006). The characteristics of the crop, which determine feeding and habitat quality, influence the growth of aphid populations (Hasken and Poehling 1995; Duffield et al. 1997; Grauby et al. 2022). On the other hand, hymenopteran parasitoids are among the most specialized natural enemies of aphids and, at some point of their life cycles, depend on the resources provided by non-crop habitats such as alternative hosts, refuges, overwintering sites (Norris and Kogan 2000; Bianchi et al. 2006) and nectar for adults to feed on during their life cycles (Bianchi and Wäckers 2008).
Although the putative effects of non-crop habitats in the landscape on biological control have received much attention in recent years, there is still much controversy regarding this question. Several authors have found that parasitoids benefit from higher proportions of semi-natural habitats at landscape scale (Chaplin-Kramer et al. 2011; Plećaš et al. 2014; Dainese et al. 2017); by contrast, a meta-analysis summarizing data from 31 countries found a similar number of positive and negative associations between pest control and semi-natural habitats in the landscape (Karp et al. 2018). However, non-crop habitats are the component of the agricultural landscapes most difficult to manage, since farmers rarely apply a management effort in off-field land (Bassa et al. 2011). Moreover, individual farmers scattered in a mosaic landscape with land properties irregularly distributed as happens in Mediterranean countries (Napoléone and Melot 2021) make more difficult a management at landscape level. Thus, the implementation of certain management strategies at field level are potentially much more interesting, but it would imply a coordination among farmers in order to modulate the risk of pests in the landscape (Begg et al. 2017).
Low-intensity practices such as organic farming benefit biological diversity, and they are a more realistic option for implementation at landscape level (Cohen and Crowder 2017). Gabriel et al. (2010) found that the aggregation of organic farming can increase the diversity of certain groups of organisms, which suggests that differences in field management—if implemented at landscape level—and in surrounding non-crop habitats could also affect the provision of ecosystem services. Conversely, some effects of cropland characteristics such as crop diversity might only become visible in resource-depleted, simplified landscapes with little semi-natural habitat cover (Redlich et al. 2018; Clemente-Orta et al. 2020).
In this study, the aim was to assess how current agricultural management at field and landscape levels affects cereal aphids and their parasitoids. The effect of two farming systems (organic/conventional) on the cereal aphid-parasitoid community was investigated by considering paired fields. To simultaneously test for the impact of the surrounding landscape scale, fields were selected across a complexity gradient characterized by the percentage of organically managed land (POML) and, secondarily, by the percentage of annually tilled arable land (PAL).
The following two hypotheses were addressed in the study: (i) cereal aphids respond mainly to field characteristics (field management system, cereal or grass cover) given that they are grass specialists and can complete their whole life cycles on cereal crops; and (ii) parasitism rates will be affected by both field (field management system, weed cover) and landscape-level variables (natural habitats, organic aggregation) given that parasitoids require resources that are present both within and outside fields to complete their life cycles.
Materials and methods
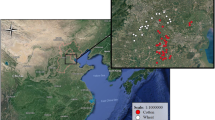
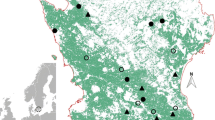
The study was carried out in 30 fields in five areas in Catalonia (NE Spain): Cabrianes (Ca), Cardona (Co), l’Espunyola (Es), Moià (Mo) and Gallecs (Ga) (Fig. 1). The set of landscapes covers an approximate area of 46 × 54 km (41°33′–42°03′ N, 1°36′–1°45′ E). Sampling sites were chosen according to their POML as one of the main objectives of this study to disentangle the effects and relative importance of organic farming aggregation in the landscape. However, owing to the scarcity of organic farms, the choice was somewhat limited and only the extent of organic farming—rather than its distinguishing features—was the landscape characteristic used for the selection of localities. These localities lay on a gradient, with two localities having a low percentage of organic arable land and two a higher percentage. Gallecs is a particular case as, when analysed with a 500-m buffer, some fields had a low POML and some a somewhat higher POML.
In each of the first four areas (Ca, Co, Es, Mo) we selected four cereal fields, two organically managed and two conventionally managed; in Ga we selected 14 fields, of which only three were organically managed. In total, 15 of the selected fields were sown with barley and the other 15 with different varieties of wheat, including three with Khorasan wheat, triticale and spelt (further details in Table S1 in Supplementary information, hereafter SI).
First, we selected the organic fields that had been managed for over a decade following organic guidelines and had been certified as organic by the Catalan Council for Organic Farming which means they use no phytosanitary products to control pests. Likewise, they use only organic fertilisers and implement diverse crop rotations including the sowing of leguminous crops. Each organic field was then paired with a control conventional field in the vicinity. Practices in conventional fields were representative of practices in many Catalan farms, where mineral fertilisers and synthetic phytosanitary products are employed. Phytosanitary products applied during the sampling season consisted of herbicides (as it is habitual for cereal crops in the area, which rarely receive other biocides). This pairing approach ensured that the conventional and organic plots shared similar pedo-climatic conditions.
Field scale
In each selected field, we established one 50-m-long transect, 3 m from and parallel to the field margin (Fig. 2). The transect was established next to unaltered field margins (e.g. not burned margins or margins where herbicides had been directly applied). Each transect was split into five 10-m-long and 1-m-wide sections, of which alternate sections (first, third and fifth) were sampled six times during the spring.
The cereal and weed cover were estimated visually by the same trained observers along each sampling segment. Crop and weed cover were recorded in each plot according to a ground cover scale (to the nearest 1% below a 10% threshold, and to the nearest 10% above that threshold). We classified weeds into three functional groups following Caballero-López et al. (2012): grasses excluding crops, forbs and legumes. Grasses can act as alternative hosts for aphids, while forbs and legumes can provide nectar resources within fields for adult parasitoids (Koricheva et al. 2000). The higher nitrogen content of legumes can attract alternative aphid hosts for parasitoids (Farooq et al. 2022).
In 2015 from late April to mid-June, once a week we collected 10 tillers as regularly distributed as possible along each segment, giving a total of 30 shoots per transect. Samplings were done between 09:30 and 18:30 h under favourable weather conditions (without rain or strong wind). We placed the collected tillers carefully inside sealable plastic bags and stored them in a portable fridge in the field. Bags were kept at 4 °C for at most four days until processed to reduce predator activity and to avoid the loss of aphids. In the days following each sampling session, we removed all aphids from the plant tillers using wet brushes. Aphids from each tiller were counted and classified by species and growth stage (nymph, adult wingless and adult winged) and preserved in 70% ethanol. Mummies were also counted, and ‘closed mummies’ from which parasitoids had not yet emerged were kept separately in individual vials, covered with a cotton plug, at room temperature (20–24 °C, with no humidity control) until the parasitoid emerged; both the parasitoid imagoes and the aphid mummies were then preserved in 70% ethanol.
All aphids (including mummies) and hatched parasitoids and most hyperparasitoids were identified to species level if possible or otherwise to genus level. The aphids were identified by Nicolás Pérez Hidalgo, the primary parasitoids (Braconidae: Aphidiinae) by José M. Michelena, and the secondary parasitoids by Mar Ferrer Suay (Cynipoidea: Figitidae: Charipinae), Emilio Guerrieri (Chalcidoidea: Encyrtidae) and Agnès Salat-Moltó (Chalcidoidea: Pteromalidae, Aphelinidae; Ceraphronoidea: Megaspilidae).
Landscape matrix
Landscape level variables (PAL, POML) were assessed in a 500-m buffer centred on each sampling transect. PAL values (mean ± SD = 74.34 ± 13.32 %; min = 48.02 %; max = 99.04 %) and the POML (mean ± SD = 23.44 ± 19.21 %; min = 0.14 %; max = 64.47 %). Arable farming (mainly cereal and legume crops) is the main land-use in these areas. Semi-natural field margins ranging from perennial grasslands to scrubland and small stands of trees were present in all areas (Salat-Moltó et al. 2023). Land-cover information was derived from the Catalan Habitats cartography adapted from the Corine Biotope Habitats (Carreras and Diego 2004), as well as from existing cartography by Caballero-López and Sans (2010). Field management information was derived from information previously gathered by the research group (José-María et al. 2010; Rotchés-Ribalta et al. 2015). ArcGIS (version 10.1) was used to calculate both parameters.
Data analysis
We summed the number of aphids and mummified aphids obtained in each section of each transect over the whole sampling period to obtain the response variables of aphid abundance and parasitism. The effect of landscape management and field scale variables on aphids and parasitoids was analysed using Generalised Linear Mixed Effects Models (GLMM), with ‘field’ nested within ‘area’ as a random effect given that samples from the same field and from the same area could be spatially dependent. As fixed factors we tested the effects of PAL and POML as continuous variables, and their interaction as descriptors of landscape management. Farming management and crop variety as categorical data and plant cover (cereal, forb, legume and grass cover) were used as continuous variables.
For the aphid abundance models, we employed a negative binomial distribution (with the default log-link function) for the error term due to the overdispersion observed during data exploration. For the parasitism rates, models included aphid abundances as an additional covariate since parasitism rates can be density-dependent (Pareja et al. 2008); we used a binomial distribution (with a logit-link function) that was appropriate for proportional data (Zuur et al. 2009).
All continuous explanatory variables were standardized before data analysis to avoid numerical issues and to facilitate the interpretation of the effects based on regression coefficients. Explanatory variables whose dispersion increased proportionally to mean values were log-transformed prior to standardization. We checked for collinearity between continuous explanatory variables and considered them to be correlated when Spearman’s correlation coefficient was greater than 0.5. We used the Student t-test to detect significant associations between our categorical and continuous explanatory variables. We found cereal and forb cover to be correlated with each other and with management and crop variety. Thus, we used a procedure similar to the one described in Zuur et al. (2009) to avoid these issues: first, we subtracted the group means for each combination of management and crop variety (four groups in total) and then divided by the overall standard deviation to maintain the relative spread of the observations between groups. While this procedure guaranteed that our continuous variables did not vary in accordance with our categorical variables, a correlation test proved that both cereal and forb cover were still correlated. We therefore subtracted cereal cover from forb cover so that both terms could be used in a linear model (Zuur et al. 2009). Furthermore, measures of legume cover were greater than zero mainly under organic management. We did not transform these measures since any standardization would have rendered the minimum levels of cover under conventional management and average levels of cover under organic management as equivalent.
We used multi-model inference to determine which variables were most important for each group (aphids and parasitoids) and to obtain an average estimate of their effects. We compared 505 models for aphid abundances and 1,024 for parasitism rates using the Akaike Information Criterion corrected for small sample sizes (AICc) (Burnham and Anderson 2002). Model averaging uses Akaike weights—assigned to each model in terms of the difference in their AICc relative to the best (lowest AICc) model—to compute a conditional weighted average of model coefficients we considered only models in which the variable was present (Burnham and Anderson 2002). Of the 512 possible models for aphid abundance, seven presented convergence problems and were thus excluded from the analysis after checking that the convergence issues did not depend on a single explanatory variable, and that such exclusion did not lead to an unbalanced representation of any variable in the model set.
We checked for the normality of residuals in our global models using graphical exploration (Zuur et al. 2009). In our global model for parasitism rates, the distribution of residuals departed slightly from normality owing to the presence of outliers. We repeated the analyses with these observations excluded and obtained normally distributed residuals; however, we found no differences in the estimated effects (direction and precision of estimates) between the models based on complete data or on subset data. Therefore, we decided to retain the models based on the complete data set.
All analyses were performed using R 3.3.2 (R Core Team 2018), with packages glmmADMB version 0.8.3.3 (Fournier et al. 2012; Skaug et al. 2016) for model fitting and MuMIn version 1.47.1 (Bartoń 2016) for multi-model inference.
Results
Aphid abundance
We collected 12,136 aphids belonging to 18 species, the most abundant being Sitobion (Sitobion) avenae (Fabricius, 1775), which represented 46% of the total aphid abundance. See Table S2 in SI for a complete list of all aphid species found.
Overall, aphids were mainly influenced by two characteristics, namely, crop variety and cereal cover, both at field level. The abundance of aphids was higher in wheat fields and in fields with less cereal cover. Neither management, landscape nor weed communities had significant effects (Fig. 3). Based on the estimated regression coefficients, the magnitudes of landscape and management effects were larger—albeit non-significantly—than those of weeds.
Multimodel-inference for the aphid abundance, showing the averaged estimates for all predictor variables and their unconditional SE for all models containing the variable (Burnham and Anderson 2002). Coefficients whose confidence intervals do not include zero are significant. Management stands for the difference Organic vs. Conventional. Crop variety represents the difference Barley vs. Wheat. Cover variables are included in the models according to the procedure described in the text
Parasitism rates
Of the 1058 parasitized aphids, 572 were reared in the laboratory. The remaining mummies were already open when collected in the field but were still used in the calculation of parasitism rates. Of the reared mummies, 267 (46%) hatched successfully. A total of 109 primary parasitoids (Hymenoptera: Braconidae: Aphidiinae) emerged, of which the most abundant species (47.7% of the total) was Aphidius uzbekistanicus Luzhetzhi 1960. We also found 133 hyperparasitoids, the most abundant species (38% of the total) being the pteromalid Pachyneuron aphidis (Bouché 1834). Twenty-five of the hatched specimens were unidentifiable. See Tables S3 and S4 in SI for a complete list of all parasitoids and hyperparasitoids found.
Parasitism rates were mainly influenced by landscape, crop characteristics and aphid abundance (Fig. 4). At landscape level, parasitism was negatively affected by PAL (lower parasitism rates where the landscape was dominated by arable land) and positively affected by POML (higher parasitism rates with a greater concentration of fields under organic management). The interaction between PAL and POML was significant, indicating that POML had no effect at high PAL. In terms of crop characteristics, crop variety—but not cereal cover—was also important in determining the rates of parasitism, which were higher in wheat and closed varieties than in barley fields. Neither field management nor the presence of weeds affected significantly parasitism rates, which were higher when aphid abundances were low.
Multimodel-inference for the parasitism rate, showing the averaged estimates for all predictor variables and their unconditional SE for all models containing the variable (Burnham and Anderson, 2002). Coefficients whose confidence intervals do not include zero are significant. Management stands for the difference Organic vs. Conventional. Crop variety represents the difference Barley vs. Wheat. Cover variables are included in the models according to the procedure described in the text
Discussion
Our study reveals that field-level factors were relevant for predicting both aphid abundances and parasitism rates, whereas, landscape variables only show a significant relationship with parasitism rates. This highlights the importance of considering simultaneously field-scale and landscape factors when analysing the aphid-parasitoid community.
Field-level factors
Crop variety is the most relevant factor explaining the variation in cereal aphid abundance. Aphid density was higher in wheat fields, which suggests a greater preference for wheat than barley. The dominance of Sitobion avenae, which was the most abundant species in the studied systems, may explain this pattern as other authors have stated (Watt 1979; Watson and Dixon 1984). They attribute the lack of interest for barley to its compact and awned ears that are less suitable than those of wheat, which may explain the smaller aphid populations found on barley in the field. Gao and Liu (2013) found that S. avenae clones performed differently on wheat and barley, with lower developmental times, higher fecundity and higher growth rates for wheat clones of S. avenae compared to barley clones. However, other studies report the opposite relationship (Sigsgaard 2002) and it seems that sampling year, cereal variety (Ba-Angood and Stewart 1980) and aphid clone diversity (Gao et al. 2014) could explain some of this variability.
Aside from crop variety, the main factor affecting aphid abundances was cereal cover. The relation between phytophagous insects and plant cover has been widely investigated (Lawton 1983; Poveda et al. 2006; Caballero-López et al. 2010) but such interactions may be either negative or positive (Evans 2008). Although many authors have addressed the topic from a plant chemistry or food quality perspective (Stiling and Moon 2005) this pattern could also be attributable to microclimatic conditions acting via insects’ physiological constraints or to the behavioural response of aphids to crop stand density. Sampaio et al. (2017) report that an increase in either precipitation or temperature favoured an increase in aphid populations. Nevertheless, high levels of precipitation combined with high temperatures did seem to act as a brake on Brassica aphid populations. Honěk et al. (2018) speculated that the negative response to plant cover was related to the microclimatic conditions in dense plots since high humidity has an adverse effect on population growth by favouring the development of mycoses. Alternatively, sparse crop stands are warmer and drier and enhance the development rate and fecundity of aphids (Honěk and Martinkova 2004). Winder et al. (2005) attributes the negative relationship with crop cover to the spread of the aphid population between more numerous shoots. This behaviour leads to the formation of smaller colonies or less persistent colonies in dense crop stands (Winder et al. 2014), which would be less detectable by our sampling method. Furthermore, greater cereal cover also implies less weed cover, thereby increasing the efficiency of host/prey searching by natural enemies and thus enhancing biological control (Gols et al. 2005); however, the analysis of parasitism rates in our study does not support this explanation.
Studies evaluating the effects of organic farming generally find positive effects across all taxa understudy (Bengtsson et al. 2005; Hole et al. 2005; Ponce et al. 2011), although variations in responses between taxa have been shown to occur (Fuller et al. 2005; Clough et al. 2007). For instance, Inclán et al. (2015) reported a significant decrease in parasitoid diversity in arable crops under conventional agriculture (because insects are negatively affected by the use of chemical pesticides) compared to organic farms where chemicals are not applied. However, conventional practices of cereal crops in a dry-land context do not commonly involve insecticide applications, which presumably could explain the lack of differences in the dichotomy organic vs. conventional agriculture in our dry-land aphid-parasitoid system. Puech et al. (2014) also attribute the lack of differences between organic and conventional management to the limited use of insecticides in conventional fields, which are not applied in a dry-land context.
The response of parasitism rate is a mix of effects from the in-field factors to landscape factors. Parasitism rate respond in the first instance to the abundance of hosts. However, the negative density dependence of parasitism on aphid abundance was unexpected, although similar to the observations made by Pareja et al. (2008) who suggest that inverse density dependence may appear when parasitoids only attack the periphery of colonies: this implies that the bigger the colony, the lower the parasitism rates. Alternatively, Hassell (1984) and Hassell et al. (1985) defended that such patterns of parasitism could be explained mechanistically in terms of the allocation of searching time in patches of different host density and the maximum attack rate per parasitoid that constrains the extent of host exploitation within a patch.
In contrast to the findings reported by Caballero-López et al. (2012), aphid abundances and parasitism rates in our study did not respond to differences in the weed community and, in fact, the size of the effect (coefficients) of all weed groups was one or two orders of magnitude less than that of other variables. One difference may stem from the fact that Caballero-López et al. (2012) sampled in the centre of fields (55 m from edges), while in our study we examined patterns that were closer to field boundaries (3 m from field edges). Just as semi-natural vegetation patches or floral strips are more relevant for natural enemies of aphids in intensive landscapes where there is a massive lack of alternative resources (Haenke et al. 2009; Jonsson et al. 2015) weeds may be more important for parasitoids further inside the field, where, compared to the edges, there are fewer available resources (José-María et al. 2011; Serée et al. 2023). Furthermore, adult parasitoids can also rely on aphid honeydew for sugar provision, although it is nutritionally inferior to floral nectar (Wäckers et al. 2008), thus making floral resources inside fields less relevant to parasitoids.
Landscape
In agreement with our initial hypothesis, we found that aphids respond mainly to field-scale parameters and do not respond to the surrounding landscape characteristics. This finding agrees with what Hawro et al. (2015) found in a cross-country comparison, where landscape complexity and agricultural intensification did not significantly affect total aphid densities. The lifestyle of aphids during the crop-growing season means they have little need to disperse until crop senescence, which thus reduces the effect of landscape on their abundances. It has been argued that field margins can be a source of field colonization by aphids early in spring (Thies et al. 2005; Plećaš et al. 2014) but this phenomenon seems to be of little relevance in a Mediterranean context where mild winters allow aphids to remain in fields during the cold season (Pons et al. 1993; Chaplin-Kramer et al. 2013).
On the other hand, our results show the relevance for parasitism rates of the landscape within a 500-m buffer. These findings are in line with a considerable body of research that supports the idea that parasitoids respond positively to landscape complexity (Chaplin-Kramer et al. 2011; Plećaš et al. 2014; Rusch et al. 2016; Dainese et al. 2017), and that parasitism rates decrease as the cropped surface increased (Thies et al. 2005; Roschewitz et al. 2005). Our results show that natural flight-capable enemies such as parasitoids are not restricted to the cropland. This is important because, if the impact of natural enemies is to be increased by promoting field margins, these margins should ideally have an impact at landscape scale rather than only providing benefits to the immediately adjacent crop (Elzinga et al. 2007). These non-crop landscape elements are necessary as Aphidiinae parasitoids have a wider resource requirement than those normally found within cereal crops (Bianchi and Wäckers 2008; Gillespie et al. 2016) and are beneficial for parasitoids even when these adjacent non-crop elements correspond with woody vegetation (Thomson and Hoffmann 2009; Salat-Moltó et al. 2023). Parasitoids may overwinter outside cropped areas and follow their hosts into the crops in spring (Landis et al. 2000; Vialatte et al. 2007; Ramsden et al. 2017).
Although the proportion of semi-natural areas was the most important determinant of the abundance of parasitoids, we also found that the proportion of organic land in the landscape played a major role, following the main headline of Galloway et al. (2021) that organic farming enhance the arthropods predators, but this depends on neighbouring patches of natural vegetation. The detected significantly higher parasitism rates with increasing POML agrees with the findings for parasitoids (Inclán et al. 2015) and for other insect groups such as butterflies, epigeal arthropods and solitary bees (Gabriel et al. 2010).
These effects may also arise because the distribution and persistence of species across landscapes depend on the species’ dispersal ability (Elzinga et al. 2007). As parasitoids have been found to respond to habitat connectivity (Fernandes et al. 2022) the amount of organic farming in agricultural landscapes appears to be a potential means of re-establishing it in farmland, therefore enhancing its parasitoid communities (Benton et al. 2003).
Conclusions
At field level, a bottom-up mechanism in which characteristics such as crop variety and crop cover influence the size of aphid populations, which in turn is the main factor affecting parasitism rates, seems to be operating. Weeds, on the other hand, play no role in either aphid or parasitoid abundances. In terms of management, field-level organic management may not be enough to affect the level of biological control exerted by parasitoids on aphids.
We can anticipate that a coordinated implementation of organic farming at landscape level is a much more promising strategy for increasing the biological control of aphids than a concentration on the individual management of fields. Any such coordinated landscape management should also include the conservation of existing non-crop habitat patches.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Ba-Angood SA, Stewart RK (1980) Occurrence, development, and distribution of cereal aphids on early and late cultivars of wheat, barley, and oats in Southwestern Quebec. Can Ent 112:615–620
Bartoń K (2016) MuMIn: Multi-Model Inference. R package version 1.47.1. https://CRAN.R.project.org/packege=MuMIn
Bassa M, Boutin C, Chamorro L, Sans FX (2011) Effects of farming management and landscape heterogeneity on plant species composition of Mediterranean field boundaries. Agric Ecosyst Environ 141:455–460
Begg GS, Cook SM, Dye R et al (2017) A functional overview of conservation biological control. Crop Prot 97:145–158
Bengtsson J, Ahnström J, Weibull AC (2005) The effect of organic agriculture on biodiversity and abundance: a meta-analysis. J Appl Ecol 42:261–269
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188
Bianchi FJJA, Wäckers FL (2008) Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biol Control 46:400–408
Bianchi FJJA, Booij CJH, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc R Soc London-Biol Sci Ser B 273:1715–1727
Bianchi FJJA, Mikos V, Brussaard L et al (2013) Opportunities and limitations for functional agrobiodiversity in the european context. Environ Sci Policy 27:223–231
Blackman RL, Eastop V (2006) Aphids on the World’s Herbaceous Plants and Shrubs. (Volume 1 Host Lists and Keys/Volume 2 The aphids). Wiley, Chichester
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference a Practical Information-Theoretic Approach. Springer-Verlag, New York [etc.]
Caballero-López B, Sans FX (2010) Cartografia d’hàbitats i d’arbres aïllats del parc de l’Espai d’Interès Natural de Gallecs (Mapa i memòria). Universitat de Barcelona, Barcelona
Caballero-López B, Blanco-Moreno JM, Pérez N et al (2010) A functional approach to assessing plant-arthropod interaction in winter wheat. Agric Ecosyst Environ 137:288–293
Caballero-López B, Blanco-Moreno JM, Pérez-Hidalgo N et al (2012) Weeds, aphids, and specialist parasitoids and predators benefit differently from organic and conventional cropping of winter cereals. J Pest Sci 85:81–88
Carreras J, Diego F (2004) Cartografia dels hàbitats a Catalunya, 1:50.000
Chaplin-Kramer R, O’Rourke ME, Blitzer EJ et al (2011) A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol Lett 14:922–932
Chaplin-Kramer R, de Valpine P, Mills NJ, Kremen C (2013) Detecting pest control services across spatial and temporal scales. Agric Ecosyst Environ 181:206–212
Clemente-Orta G, Madeira F, Batuecas I et al (2020) Changes in landscape composition influence the abundance of insects on maize: the role of fruit orchards and alfalfa crops. Agric Ecosyst Environ. https://doi.org/10.1016/j.agee.2019.106805
Clough Y, Holzschuh A, Gabriel D et al (2007) Alpha and beta diversity of arthropods and plants in organically and conventionally managed wheat fields. J Appl Ecol 44:804–812
Cohen AL, Crowder DW (2017) The impacts of spatial and temporal complexity across landscapes on biological control: a review. Curr Opin Insect Sci 20:13–18
Dainese M, Montecchiari S, Sitzia T et al (2017) High cover of hedgerows in the landscape supports multiple ecosystem services in Mediterranean cereal fields. J Appl Ecol 54:380–388
Dainese M, Martin EA, Aizen MA et al (2019) A global synthesis reveals biodiversity-mediated benefits for crop production. Sci Adv. https://doi.org/10.1126/sciadv.aax0121
Duelli P, Obrist MK (2003) Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl Ecol 4:129–138
Duffield SJ, Bryson RJ, Young JEB et al (1997) The influence of nitrogen fertiliser on the population development of the cereal aphids Sitobion avenae (F) and metopolophium dirhodum (wlk) on field grown winter wheat. Ann Appl Biol 130:13–26
Elzinga JA, van Nouhuys S, van Leeuwen DJ, Biere A (2007) Distribution and colonisation ability of three parasitoids and their herbivorous host in a fragmented landscape. Basic Appl Ecol 8:75–88
Eurostat (2022) European Statistics. https://ec.europa.eu/eurostat/. Accessed 22 Dec 2022
Evans EW (2008) Multitrophic interactions among plants, aphids, alternate prey and shared natural enemies—a review. Eur J Entomol 105:369–380
Farooq MO, Razaq M, Shah FM (2022) Plant diversity promotes species richness and community stability of arthropods in organic farming. Arthropod Plant Interact 16:593–606
Fernandes LD, Mata AS, Godoy WAC, Reigada C (2022) Refuge distributions and landscape connectivity affect host-parasitoid dynamics: motivations for biological control in agroecosystems. PLoS ONE 17:e0267037
Fournier DA, Skaug HJ, Ancheta J et al (2012) AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw 27:233–249
Fuller RJ, Norton LR, Feber RE et al (2005) Benefits of organic farming to biodiversity vary among taxa. Biol Lett 1:431–434
Gabriel D, Sait SM, Hodgson JA et al (2010) Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecol Lett 13:858–869
Galloway AD, Seymour CL, Gaigher R, Pryke JS (2021) Organic farming promotes arthropod predators, but this depends on neighbouring patches of natural vegetation. Agric Ecosyst Environ 310:107295
Gao S, Liu D (2013) Differential performance of Sitobion avenae (Hemiptera: Aphididae) clones from wheat and barley with implications for its management through alternative cultural practices. J Econ Entomol 106:1294–1301
Gao S-X, Liu D-G, Chen H, Meng X-X (2014) Fitness traits and underlying genetic variation related to host plant specialization in the aphid Sitobion avenae. Insect Sci 21:352–362
Gillespie MAK, Gurr GM, Wratten SD (2016) Beyond nectar provision: the other resource requirements of parasitoid biological control agents. Entomol Exp Appl 159:207–221
Gols R, Bukovinszky T, Hemerik L et al (2005) Reduced foraging efficiency of a parasitoid under habitat complexity: implications for population stability and species coexistence. J Anim Ecol 74:1059–1068
González-Díaz L, van den Berg F, van den Bosch F, González-Andújar JL (2012) Controlling annual weeds in cereals by deploying crop rotation at the landscape scale: Avena sterilis as an example. Ecol Appl 22:982–992
Grauby S, Ferrer A, Tolon V et al (2022) Can mixed intercropping protect cereals from aphid-borne viruses? An experimental approach. Insects 13:1–14
Gray S, Gildow FE (2003) Luteovirus-aphid interactions. Annu Rev Phytopathol 41:539–566
Haenke S, Scheid B, Schaefer M et al (2009) Increasing syrphid fly diversity and density in sown flower strips within simple vs. complex landscapes. J Appl Ecol 46:1106–1114
Hasken KH, Poehling HM (1995) Effects of different intensities of fertilisers and pesticides on aphids and aphids predators in winter wheat. Agric Ecosyst Environ 52:45–50
Hassell MP (1984) Parasitism in patchy environments: inverse density dependence can be stabilizing. J Math Appl Med Biol 1:123–33. https://doi.org/10.1093/imammb/1.1.123
Hassell MP, Lessells CM, McGavin GC, (1985) Inverse density dependent parasitism in a patchy environment: a laboratory system. Ecol Entomol 10:393–402. https://doi.org/10.1111/j.1365-2311.1985.tb00737.x
Hawro V, Ceryngier P, Tscharntke T et al (2015) Landscape complexity is not a major trigger of species richness and food web structure of european cereal aphid parasitoids. Biocontrol 60:451–461
Hole DG, Perkins AJ, Wilson JD et al (2005) Does organic farming benefit biodiversity? Biol Conserv 122:113–130
Holland JM, Douma JC, Crowley L et al (2017) Semi-natural habitats support biological control, pollination and soil conservation in Europe. A review. Agron Sustain Dev. https://doi.org/10.1007/s13593-017-0434-x
Honěk A, Martinkova Z (2004) Host plant age and population development of a cereal aphid, Metopolophium dirhodum (Hemiptera: Aphididae). Bull Entomol Res 94:19–26
Honěk A, Martinkova Z, Saska P, Dixon AFG (2018) Aphids (homoptera: Aphididae) on winter wheat: predicting maximum abundance of Metopolophium dirhodum. J Econ Entomol 111:1751–1759
Inclán DJ, Cerretti P, Gabriel D et al (2015) Organic farming enhances parasitoid diversity at the local and landscape scales. J Appl Ecol 52:1102–1109
Jonsson M, Straub CS, Didham RK et al (2015) Experimental evidence that the effectiveness of conservation biological control depends on landscape complexity. J Appl Ecol 52:1274–1282
José-María L, Armengot L, Blanco-Moreno JM et al (2010) Effects of agricultural intensification on plant diversity in Mediterranean dryland cereal fields. J Appl Ecol 47:832–840
José-María L, Blanco-Moreno JM, Armengot L, Sans FX (2011) How does agricultural intensification modulate changes in plant community composition? Agric Ecosyst Environ 145:77–84
Karp DS, Chaplin-Kramer R, Meehan TD et al (2018) Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc Natl Acad Sci USA 115:E7863–E7870
Koricheva J, Mulder CPH, Schmid B et al (2000) Numerical responses of different trophic groups of invertebrates to manipulations of plant diversity in grasslands. Oecologia 125:271–282
Landis DA, Wratten SD, Gurr GM (2000) Habitat managment to conserve natural enemies of arthropods pests in agriculture. Annu Rev Entomol 45:175
Lawton JH (1983) Plant architecture and the diversity of phytophagous insects. Annu Rev Entomol 28:23–39
Napoléone C, Melot R (2021) Farmland management and sustainable development in the Mediterranean: land use changes, public policies, and collective resources. Reg Environ Chang 21:31
Norris RF, Kogan M (2000) Interaction between weeds, arthropods pests, and their natural enemies in managed ecosystems. Weed Sci 48:94–158
Pareja M, Brown VK, Powell W (2008) Aggregation of parasitism risk in an aphid-parasitoid system: effects of plant patch size and aphid density. Basic Appl Ecol 9:701–708
Plećaš M, Gagić V, Janković M et al (2014) Landscape composition and configuration influence cereal aphid–parasitoid–hyperparasitoid interactions and biological control differentially across years. Agric Ecosyst Environ 183:1–10
Ponce C, Bravo C, García de León D et al (2011) Effects of organic farming on plant and arthropod communities: a case study in Mediterranean dryland cereal. Agric Ecosyst Environ 141:193–201
Pons X, Comas J, Albajes R (1993) Overwintering of cereal aphids (Homoptera: Aphididae) on durum wheat in a Mediterranean climate. Environ Entomol 22:381–387
Poveda K, Steffan-Dewenter I, Scheub S, Tscharntke T (2006) Belowground effects of organic and conventional farming on aboveground plant–herbivore and plant–pathogen interactions. Agric Ecosyst Environ 113:162–167
Puech C, Baudry J, Joannon A et al (2014) Organic vs. conventional farming dichotomy: does it make sense for natural enemies? Agric Ecosyst Environ. https://doi.org/10.1016/j.agee.2014.05.002
Ramsden M, Menendez R, Leather S, Wäckers F (2017) Do natural enemies really make a difference? Field scale impacts of parasitoid wasps and hoverfly larvae on cereal aphid populations. Agric For Entomol 19:139–145
R Core Team (2018) R: A language and environment for statistical computing
Redlich S, Martin EA, Steffan-Dewenter I (2018) Landscape-level crop diversity benefits biological pest control. J Appl Ecol 55:2419–2428
Roschewitz I, Hücker M, Tscharntke T, Thies C (2005) The influence of landscape context and farming practices on parasitism of cereal aphids. Agric Ecosyst Environ 108:218–227
Rotchés-Ribalta R, Blanco-Moreno JM, Armengot L et al (2015) Both farming practices and landscape characteristics determine the diversity of characteristic and rare arable weeds in organically managed fields. Appl Veg Sci 18:423–431
Rusch A, Chaplin-Kramer R, Gardiner MM et al (2016) Agricultural landscape simplification reduces natural pest control: a quantitative synthesis. Agric Ecosyst Environ 221:198–204
Salat-Moltó A, Caballero-López B, Pérez-Hidalgo N et al (2023) Not all field margins are equally useful: effects of the vegetation structure of margins on cereal aphids and their natural enemies. Insects. https://doi.org/10.3390/insects14020156
Sampaio MV, Korndörfer AP, Pujade-Villar J et al (2017) Brassica aphid (Hemiptera: Aphididae) populations are conditioned by climatic variables and parasitism level: a study case of Triângulo Mineiro, Brazil. Bull Entomol Res 107:410–418
Serée L, Barbottin A, Chiron F et al (2023) Within-field floral resources have the potential to increase parasitism rates in winter oilseed rape pests more than resources at field margins. Agric Ecosyst Environ 344:108288
Sigsgaard L (2002) A survey of aphids and aphid parasitoids in cereal fields in Denmark, and the parasitoids’ role in biological control. J Appl Entomol 126:101–107
Skaug H, Fournier D, Bolker B et al (2016) glmmADMB: Generalized Linear Mixed Models using “AD Model Builder”. R package version 0.8.3.3
Steffan-Dewenter I, Münzenberg U, Tscharntke T (2001) Pollination, seed set and seed predation on landscape scale. Proc R Soc London- Biol Sci Ser B 268:1685–1690
Stiling P, Moon DC (2005) Quality or quantity: the direct and indirect effects of host plants on herbivores and their natural enemies. Oecologia 142:413–420
Thies C, Tscharntke T (1999) Landscape structure and biological control in agroecosystems. Sci (80-) 285:893–895
Thies C, Roschewitz I, Tscharntke T (2005) The landscape context of cereal aphid-parasitoid interactions. Proc Biol Sci 272:203–210
Thomson LJ, Hoffmann AA (2009) Vegetation increases the abundance of natural enemies in vineyards. Biol Control 49:259–269
Vialatte A, Plantegenest M, Simon J-C, Dedryver C-A (2007) Farm-scale assessment of movement patterns and colonization dynamics of the grain aphid in arable crops and hedgerows. Agric For Entomol 9:337–346
Vickerman GP, Wratten SD (1979) The biology and pest status of cereal aphids (Hemiptera: Aphididae) in Europe: a review. Bull Entomol Res 69:1–32
Vollhardt IMG, Bianchi FJJA, Wäckers FL et al (2010) Spatial distribution of flower vs. honeydew resources in cereal fields may affect aphid parasitism. Biol Control 53:204–213
Wäckers FL, van Rijn PCJ, Heimpel GE (2008) Honeydew as a food source for natural enemies: making the best of a bad meal? Biol Control 45:176–184
Watson SJ, Dixon AFG (1984) Ear structure and the resistance of cereals to aphids. Crop Prot 3:67–76
Watt AD (1979) The effect of cereal growth stages on the reproductive activity of Sitobion avenue and metopolophium dirhodum. Ann Appl Biol 91:147–157
Winder L, Griffiths GJK, Perry JN et al (2005) The role of large-scale spatially explicit and small-scale localized processes on the population dynamics of cereal aphids. Bull Entomol Res 95:579–587
Winder L, Alexander CJ, Woolley C et al (2014) Cereal aphid colony turnover and persistence in winter wheat. PLoS ONE 9:e106822
Zuur AF, Ieno EN, Walker NJ et al (2009) Mixed Effects Models and Extensions in Ecology with R. Springer, New York
Acknowledgements
We would like to thank both the farmers and the Consortium of the Parc de l’Espai d’Interès Natural de Gallecs for their cooperation and access to their fields. We are also grateful to Juli Pujade-Villar for his help with the identification of Pteromalidae (Hymenoptera: Chalcidoidea). Thanks also to Sergi Gago for his assistance with references.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was partially supported by the MICINN 2018 project (RTI2018-095597-B-I00) from the Spanish Ministry of Science and Innovation and the project AGRIBIOPOL (CGL2012-39442) from the Spanish Ministry of Economy, Industry and Competitiveness. A.S.-M. was supported by an APIF fellowship by the University of Barcelona. Mike Lockwood performed the linguistic revision, which was funded by the Museu de Ciències Naturals de Barcelona.
Author information
Authors and Affiliations
Contributions
AS, BC, and JMB contributed to the study conception and design. All authors performed the experimental work. AS, NP, JMM, MF and EG studied the sampled specimens. AS, and JMB performed the computational analyses and worked on the visualisation. AS, BC, and JMB wrote the first draft, which includes input from all co-authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salat-Moltó, A., Blanco-Moreno, J.M., Pérez Hidalgo, N. et al. Aggregation of organically managed fields promotes aphid parasitism in cereal crops under Mediterranean conditions. Landsc Ecol 38, 3555–3567 (2023). https://doi.org/10.1007/s10980-023-01715-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-023-01715-w