Abstract
Context
Habitat loss and alteration affect wildlife populations worldwide. Bush encroachment alters landscapes and threatens arid and semi-arid grasslands, but its effects on predator–prey relationships and carnivore community ecology are not well understood. Predation strategies of large predators, for example high-speed pursuits versus ambush from short distances, are likely to be affected differently by bush encroachment.
Objectives
We assessed how bush encroachment affects overall predation success of cheetahs (Acinonyx jubatus) and leopards (Panthera pardus) in a savanna landscape under variable fractional woody cover (FWC). We tested if predation success remained relatively unchanged for leopards across a gradient of FWC, and whether cheetah predation was most successful at low to intermediate cover and varied seasonally.
Methods
Belly scores of predators were measured from camera-trap images collected over 7 years in north-central Namibia and used to index predation success. We derived predicted belly score probabilities as a function of FWC, which was measured using a Synthetic Aperture Radar (SAR)-derived satellite data layer.
Results
Predicted leopard predation success was highest at 0.27–0.34 FWC in the dry season, potentially due to lower prey density in highly covered areas and decreased prey catchability in low cover. Predicted cheetah predation success was highest at 0.24–0.28 FWC in the wet season, potentially due to increased landscape openness, high availability of habitat margins for visualising and stalking prey, and decreased kleptoparasitism by leopards. These results highlight optimal habitat cover thresholds that favour lower FWC for cheetah than for leopard.
Conclusions
The findings indicate that landscape heterogeneity is important for predation success of cheetahs and leopards, suggesting that habitat management should focus on bush control efforts to maintain intermediate levels of bush cover.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat conditions influence habitat selection by both predator and prey (Gorini et al. 2012). For prey, these conditions determine predation risk and the availability and quality of food and shelter (Heithaus 2001). Habitat with low quality or limited forage may be avoided (Bjørneraas et al. 2012), leading to variation in prey densities (Kiffner et al. 2017). In habitats with high vegetation cover, accessibility of prey to predators may be decreased for smaller prey that are able to find refuge (Loggins et al. 2019), but increased for larger prey for which refuges are lacking or non-existent and escape routes are obstructed (Martin and Owen-Smith 2016). Furthermore, habitat condition can determine predation success of carnivores directly, through alterations in hunting grounds, or indirectly, through impacts on prey, depending on the predation strategy used (Hebblewhite et al. 2005; Balme et al. 2007).
Bush encroachment, whereby growth of herbaceous vegetation is suppressed by encroaching woody species, threatens grasslands and savannas globally (Singh and Joshi 1979; Hobbs and Mooney 1986; Jeltsch et al. 1997), particularly in arid and semi-arid climates (De Klerk 2004). Various factors may promote bush encroachment, including overgrazing (Walter 1964), climate change (Bond and Midgley 2000), fire suppression (Scheiter and Higgins 2009) and the elimination of mega-herbivores (O'connor et al. 2014). Bush encroachment may lead to decreased herbivore species richness (Soto-Shoender et al. 2018) through reduced grazing areas and lower habitat productivity (Oba et al. 2000), and numbers of some smaller prey species may be further depleted in the dry season due to decreased food availability (Massawe et al. 2011). Also, since open areas enable increased visibility, potentially allowing early predator detection (Riginos and Grace 2008; Le Roux et al. 2018), diversity and population density of large herbivore species may decrease as habitat transitions from open to woody-dominated. This may lead to alterations in the configuration of the “landscape of fear” (Laundré et al. 2001).
In Namibia, bush encroachment poses a major threat to biodiversity as it contributes towards land degradation (De Klerk 2004). The cheetah (Acinonyx jubatus) and leopard (Panthera pardus) are top predators that coexist across Namibian farmlands. Due to the extirpation of other top predators, including the lion (Panthera leo) and spotted hyena (Crocuta crocuta), from many Namibian farms (Marker and Dickman 2005), competitive pressure in the large carnivore guild is somewhat reduced. The leopard, as an ambush predator, may directly benefit from increased bush cover providing greater ambush opportunities (Davies et al. 2016). However, this might only be advantageous for leopards below a certain threshold of bush encroachment, the value of which remains unclear. Contrastingly, as a cursorial predator, the cheetah may benefit less or may be negatively impacted by bush encroachment due to a reliance on open areas for high-speed pursuits. However, Bissett and Bernard (2007) revealed that cheetahs can successfully hunt in habitats characterised by thicket vegetation and noted decreased kleptoparasitism (stealing of kills by other predators) therein, as was also described by Mills et al. (2004). It is therefore unclear how bush encroachment affects overall predation success of cheetahs and leopards, but as leopards have shown high adaptability to a wide range of habitats (Stein and Hayssen 2013) they may respond better to the associated changes in habitat conditions and prey densities. Such information is needed to understand the response of carnivore guilds to future habitat change given predicted increases in bush encroachment (Hoffman et al. 2019).
The ability of top predators to achieve high satiation in bush-encroached landscapes is likely to be affected by numerous factors. For example, cheetahs have been shown to frequently use areas near water sources, where prey encounter rates are often higher (Rostro-García et al. 2015), and to select for habitats in protected areas (Klaassen and Broekhuis 2018). Understanding factors that combine to affect predation success may be particularly important for subordinate predators like the cheetah, which could be exposed to the cumulative effects of being out-competed by dominant predators and forced to hunt in unsuitable habitats. Assessment of predation success could provide early warning of detrimental effects of habitat change on predator population fitness. Belly scoring methods can be used to estimate satiation in wild carnivores (Knobel et al. 2002; Potgieter and Davies-Mostert 2012) immediately or soon after a feeding event and thereby provide a means of measuring predation success. Historically, these scores were based on an ordinal scale and obtained via direct observation (Bertram 1975; Caro 1987). More recently, belly scores have been obtained from photographs and calculated remotely (Potgieter and Davies-Mostert 2012). Camera-trapping is a non-invasive surveying method that can provide images of elusive species in the wild (Fabiano et al. 2018) and may be used to obtain belly measurements. To minimise observer bias and variability arising from subjective belly scoring methods, objective and quantitative techniques that are easily repeatable can be used (Schiffmann et al. 2017; Cloutier 2020).
To assess whether predation success differs between habitats in a bush-encroached African savanna, this study used an objective, quantitative method to measure belly sizes of leopards and cheetahs from camera-trap images collected over 7 years in north-central Namibia. Intraspecific belly score comparisons were made spatially, using a 2016 Synthetic Aperture Radar (SAR)-derived snapshot showing fractional woody vegetation cover (FWC), and by season (wet/dry). The SAR-derived FWC layer was generated using remote sensing and is indicative of bush encroachment (Wessels et al. 2019). We also evaluated whether distance to water point and proportion of protected area around the camera trap locations that photographed leopards and cheetahs were associated with predation success. We tested following hypotheses:
-
(1)
leopard predation success has low variability with changing FWC, as leopards can adapt well in a range of environments (Nowell and Jackson 1996) and are the dominant predators in the study system;
-
(2)
cheetah predation success is highest in low to intermediate FWC, as cheetahs can successfully hunt in open landscapes and tolerate some level of thicket vegetation, but face potential kleptoparasitism by leopards in the latter (Bissett and Bernard 2007);
-
(3)
predation success of leopards and cheetahs is higher close to water, as water sources are predictable locations to encounter and kill prey (Valeix et al. 2010; Constant et al. 2015);
-
(4)
both predators have higher predation success at high proportion of protected area due to high prey abundance on protected land, because prey abundance is a critical driver of large carnivore distribution (Hayward et al. 2007; Winterbach et al. 2013);
-
(5)
leopard and cheetah predation success are lower in the dry season due to reductions in numbers of some prey (Ogutu and Owen-Smith 2003; Massawe et al. 2011).
Materials and methods
Study area
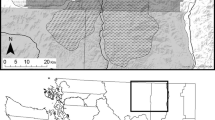
We selected the Waterberg Conservancy in north-central Namibia (20.43709 S, 17.01620 E; Fig. 1) as the study area. The climate is semi-arid, with daily temperatures highest in January (x̄ = 22.7 °C) and lowest in July (x̄ = 13.4 °C; Mendelsohn et al. 2002). Seasons are categorised into wet (November–April), within which the average annual rainfall of 400–500 mm is mostly concentrated, and dry (May–October; Mendelsohn et al. 2002). The area is mostly flat and the dominant vegetation is thornbush savanna (Barnard 1998), where bush encroachment currently poses a threat (SAIEA 2016; Nghikembua et al. 2021). The main land use practices are livestock farming, game ranching, eco-tourism and trophy hunting of herbivores and some carnivores for sport and animal display as well as for the meat (Marker-Kraus et al. 1996). Water points include artificial dams and water troughs maintained for livestock and/or wildlife. No permanent streams or natural lakes exist in this area. The presence of semi-permanent waterpoints promotes a largely sedentary prey base (Marker and Dickman 2005) comprised of species like kudu (Tragelaphus strepsiceros), steenbok (Raphicerus campestris), duiker (Sylvicapra grimmia), eland (Taurotragus oryx), springbok (Antidorcas marsupialis), oryx (Oryx gazelle), warthog (Phacochoerus africanus) and scrub hare (Lepus saxatilis). Carnivores other than leopard and cheetah include brown hyena (Hyaena brunnea), caracal (Caracal caracal), serval (Leptailurus serval) and black-backed jackal (Canis mesomelas). Lions, spotted hyenas and wild dogs (Lycaon pictus) are absent (Marker and Dickman 2005).
Map created in Q-GIS 3.10 showing the layout of scent-marking posts, waterpoints, buffers and protected area, with the study area location in Namibia shown on inset map. Buffers were selected for each species as those with the lowest AICc during AICc ranking of linear models in R; final buffers (3.065 km radius) for cheetah are based on 12 h digestion time and 6.13 km daily movement rate (Houser et al. 2009); final buffers for leopard (2.07 km radius) are based on 36 h digestion time and 1.38 km daily movement rate (Marker and Dickman 2005). Raster FWC layer provided by Wessels et al. (2019). Data for layers provided by CCF
Data collection
We selected camera trap images from an existing database established by the Cheetah Conservation Fund (CCF) as part of long-term monitoring of wildlife in the Waterberg Conservancy. Camera trap surveys were carried out across CCF land and commercial farms using Digital Infrared Bushnell® Trophy Cam™ (Kansas, USA) cameras, which were set up according to methods outlined in Fabiano et al. (2020) and checked weekly. These images had been collected to estimate cheetah density and monitor cheetah activity and were available for 2013–2014 and 2017–2019. Although images were available prior to 2013 and after 2019, we focused on the 7-year period of 2013–2019 as it allowed analysis of images taken ± 3 years from the year (2016) when Synthetic Aperture Radar (SAR)-derived woody vegetation data was collected for this region. Leopards and cheetahs in the study area share scent-marking posts, which consist mainly of large trees and sometimes prominent termite mounds (Walker et al. 2016; Verschueren et al. 2021). We selected images from camera traps placed at 21 scent-marking posts, which were all the marking sites that we knew of in the study area (Fig. 1). Analysing images from other locations may have given larger belly scores unrelated to predation success, for example immediately after the animal consumed water at a waterhole (scent-marking posts are mostly > 1 km from waterpoints) or during pregnancy. Pregnant female cheetahs are unlikely to visit mark-trees (Cornhill and Kerley 2020). Pregnant female leopards are not expected to visit scent posts because scent marking in leopards and other felids is generally a sign of oestrus (Sunquist and Sunquist 2002; Balme et al. 2013), and pregnant females presumably do not need to find mates. We could not identify individuals using spot or rosette patterns as most photos were captured at night and cameras were not equipped with white flash. Because images were taken in bursts of three per trigger with 30-s intervals between triggers, to prevent pseudo-replication we considered images acquired > 30 min apart to reflect independent events (O’Brien et al. 2003). Cheetah sex can be difficult to estimate from camera trap data and adult female leopards are difficult to distinguish from subadult males (Balme et al. 2012); we could only identify testicles for 28% and 20% of measurable images for leopards and cheetahs, respectively. Therefore, we pooled data within species for analyses. For images showing females with young, we only measured the adult female. As male cheetah coalitions hunt as a group (Hunter and Hamman 2003), we assumed that one belly score represented predation success for the whole group and took measurements from one male only. To prevent selection bias, we selected the male showing the best positioning in relation to the camera.
Belly scores
We calculated belly scores following a quantitative belly score method adapted after Cloutier (2020). This aimed to reduce observer bias from subjective categorical estimations. Following a quantitative belly score method adapted after Cloutier (2020), we took three morphometric measurements from each image: (1) front leg (FL) from the highest dorsal point of the scapula of the front leg to the lateral epicondyle of the same leg; (2) belly chord length (BCL) from the distal point of the sacroiliac process to the front leg armpit, along the base of the floating ribs; (3) belly drop (BD) from the base of the interface between the floating ribs and the true (sternum-attached) ribs, down to the base of the belly (Fig. 2). We determined images as suitable for measurement if they displayed a lateral view of the animal with the axilla, belly, front leg and sacroiliac process visible and if stance was perpendicular to the camera view; images where stance was greater than 10 degrees towards or away from the camera were eliminated to prevent measurement inaccuracies. Horizontal chord (HC) and vertical chord (VC) angles (Fig. 2) were provided automatically for FL and BCL measurements, respectively, and we used these to obtain the overall front leg angle (Θ; Table S1). We calculated measurements and angles using GIMP 2.10.24 (GIMP Development Team 2021), which allowed image rotation, magnification and editing to enhance visibility. To standardise leg angles across various postures, we measured HC from an image showing near-perfect posture, where the animal was standing on a level surface with all feet in contact with the ground, the neck unstretched and the back flat (e.g. Figure 2). We subtracted these near-perfect HC angles (83.94° for leopard, 81.01° for cheetah) from Θ for all images to obtain adjusted leg angles (Δ). We then included adjusted leg angles in a standardised belly score formula (belly drop/standardised BCL; Table S1) and used this belly score as a proxy for predation success. All calculations were performed in MS Excel.
An example camera-trap image of a cheetah showing morphometric points used to derive belly score measurements (in pixels) and angles (in degrees). FL front leg length, BCL belly chord length, BD belly drop length, HC horizontal chord angle, VC vertical chord angle, Θ leg angle calculated from HC and VC. Measurements in this image were used as the standard for cheetah as the image displayed near-perfect posture. Image provided by CCF. Belly score method adapted from Cloutier (2020). Image created in GIMP 2.10.24
Bush encroachment
We compiled GIS data corresponding to the camera trap (scent-marking post) locations (Table 1) and used QGIS 3.10 (QGIS Development Team 2019) to generate raster layers for analyses. We measured land cover in QGIS using a fractional woody cover (FWC) raster layer provided by Wessels et al. (2019), who defined FWC as the proportion of a 50 m × 50 m [raster] pixel occupied by woody vegetation. The FWC layer was created by Wessels et al. (2019) using remote sensing and Synthetic Aperture Radar (SAR)-derived satellite data, which provided greater woody vegetation detail than existing maps for the area. We calculated the mean FWC (mFWC) for circular buffers around camera-traps and used these values to infer the level of bush encroachment corresponding to camera trap images. The main purpose of the buffers was to capture the area that included the predation event (i.e., capture/subdue of prey). The FWC of the actual predation site is not critical to know if the mean FWC in the general vicinity is known. Although the buffers are meant to encompass the kill location, they have additional management relevance because they likely comprise areas for predation stages more broadly (e.g., search and detection of prey), and areas that are perceived by the predators as safe for prey consumption.
We set buffers at radii calculated as the mean movement rate (kmph) multiplied by digestion time (DT; h) for each species. As data showing the time taken for belly size to decrease after a large meal was not available in the literature for large, free-ranging felids, we included digestion times in 12-h increments between 12 and 72 h (Borstlap 2002; Table S2) in our initial analysis. Reliable data detailing daily cheetah movement rates was not available for the study area. Therefore, we used the estimated daily movement rate from a study in Botswana, where climate and habitat were comparable and where farmland also constituted a large proportion of cheetah home range (Houser et al. 2009); we calculated mean hourly movement rate from this estimate for male cheetahs (Table S2), as male cheetahs comprise most scent-mark post visits (Cornhill and Kerley 2020). Male and female leopards in our study area have been observed to visit scent-marking posts at approximately equal frequencies (CCF, unpublished data). Therefore, we obtained a mean hourly movement rate using an estimate by Marker and Dickman (2005) for the study region and averaged this between male and female leopards (Table S2). We created FWC raster layers for each buffer (radii given in Table S2) using the QGIS “Clip raster by mask layer” tool and calculated mFWC for each buffer using Zonal statistics. We calculated distance to the nearest water point (distWP) for each camera trap location in QGIS. We also calculated the proportion of protected area (pPA) within buffers by dividing the pixel count (30 m × 30 m resolution) of protected area by the total pixel count within buffers, which were provided by QGIS Zonal statistics.
Data analysis
Using variables outlined in Table 1 we ran analyses in RStudio 1.4.1717 (RStudio Team 2020). To assess the influence of measured parameters on belly scores, we ran linear regression using the R lm function for the following sets of models: leopard wet season (LWS), leopard dry season (LDS), cheetah wet season (CWS) and cheetah dry season (CDS). We tested non-log and log-transformed belly scores against both linear and quadratic mFWC. We then ranked the models using Akaike’s Information Criterion adjusted for small sample size (AICc; Burnham and Anderson 2002) to identify whether these transformations led to better fitted models. Non-log belly scores and quadratic mFWC gave better fitting models, so we included these variables in AICc ranking with buffers to determine the best buffer radius for each species-season set. Selected buffer radii were, for both seasons, 2.07 km (36 h DT) for leopard and 3.065 km (12 h DT) for cheetah (Fig. 1). We did not include variables that were highly correlated (r >|0.6|) in the same models, except the linear and quadratic FWC buffer covariates that are inherently highly correlated. We used ΔAICc to rank models within each set (Tables S3–S6). To further assess for multicollinearity, we calculated Variance Inflation Factors (VIF) using the vif function from the R car library (Fox and Weisberg 2019) for supported models (ΔAICc < 2 and ΔAICc < ΔAICcNull). We selected models with low collinearity (VIF < 4; Chatterjee et al. 2000) for interpretation. To enable interpretation of an association between belly score and quadratic mFWC, we plotted predicted belly score probabilities against mFWC for top ranking models using the R package ggplot2 (Wickham 2016). We also plotted predicted probabilities for all covariates in the best models.
Results
From the total number of independent events for leopard (n = 295) and cheetah (n = 221), we selected 120 images as suitable for belly score measurement for leopard and 94 for cheetah (summary statistics given in Table 2).
Leopard
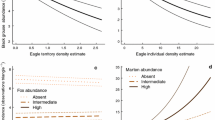
Only the dry season model set contained supported belly score models for leopard (ΔAICc < 2 and ΔAICc < ΔAICcNull) (Table S4). The model that received the most support (LDS5) contained the linear and quadratic FWC, as well as distance to waterpoints (distWP; Tables 3, S7). This multivariate model was statistically significant (F3,22 = 4.17, P = 0.018, R2adj = 0.276) and revealed that there was a higher probability of larger belly scores being detected at intermediate mFWC (Table 4; Fig. 3a). Belly scores were also greater further from waterpoints (Table 4; Fig. 3b).
Graphs created in RStudio showing predicted probabilities of leopard (a, b) and cheetah (c, d) belly score against covariates in the two top models (Table 4). Plots for covariates in the top leopard dry season model are: a quadratic mFWC; b distWP. Plots for covariates in the top cheetah wet season model are: c quadratic mFWC; d pPA. No models were supported for leopard in the wet season or cheetah in the dry season. Covariates for which confidence intervals did not overlap zero are indicated by an asterisk
Cheetah
Cheetah belly score models received support only for the wet season (Table S5) and, similar to leopard, the model that received the most support (CWS7; F3,78 = 3.83, P = 0.013, R2adj = 0.095) featured the FWC covariates, but also proportion of protected area (pPA; Tables 3, S8). Belly scores were larger at intermediate FWC (Table 4; Fig. 3c) and marginally greater at higher pPA (Table 4; Fig. 3d). Despite the contribution of distWP and pPA in supported multivariate models, the only univariate model that was supported for cheetah in the wet season was the FWC model (Table S5). For the dry season, the sample size was too small to enable model convergence for the most complex model (Table S6).
Discussion
Inferred predation success was highest at intermediate woody cover for leopard (0.27–0.34 FWC; Fig. 3a) in the dry season and for cheetah (0.24–0.28 FWC; Fig. 3c) in the wet season. Inferred predation also increased with greater distance from waterpoints for leopard and marginally so with higher proportion of protected area for cheetah. As support was only received for models in one season for each species, we were unable to address hypothesis (5) relating to seasonal differences in predation success.
Results from the best fitting leopard dry season model did not support hypothesis (1), that leopard predation success remains relatively unchanged with changing FWC. Previous studies suggest that leopard habitat preference may be determined largely by prey density (Marker and Dickman 2005; Stein et al. 2011). Prey density may be high in areas of low cover due to increased visibility of predators and increased likelihood of escape leading to decreased prey catchability (Balme et al. 2007). Our placement of camera traps at scent marking trees used by cheetah and leopard did not allow estimation of prey availability, because prey generally avoid scent marking sites of predators to decrease encounter risk (Apfelbach et al. 2005). Leopards may follow prey to areas with low cover, as supported by Nghikembua et al. (2020) who noted significantly increased leopard detection and large and medium ungulate availability in habitats that had undergone bush-thinning. However, a reliance on ambush predation and short strike distances may lead to lower leopard predation success here. Prey catchability may also be lower in high cover where bush encroachment is extensive due to decreased detection of prey by the predator (Balme et al. 2007) and the presence of impenetrable thickets impeding attack.
A complicating factor is that these predators may feed on small prey that may be common in areas of high cover (e.g. Crowell et al. 2016). Consumption of small prey may lead to adequate or even high satiation, but belly scores of predators that have consumed small prey may not accurately reflect satiation if smaller prey are more quickly digested (Martinussen and Båmstedt 1999). This could have lead to inaccuracies in estimates of predation success. However, the majority of prey species consumed by cheetahs and leopards are of medium or large size (Hayward et al. 2006a, b), which likely limited variation in belly score estimates resulting from variable prey size-related digestion times.
Differences in predation strategy between leopard and cheetah are only partially reflected by the difference in FWC thresholds for the two species, with predation success of the cheetah, a cursorial predator, predicted to be greater at lower FWC, but only marginally so (Fig. 3). Results for cheetah are mostly supportive of hypothesis (2), that cheetah predation success would be higher at low to intermediate FWC, which supports findings from Gros and Rejmánek (1999). As highlighted by Nghikembua et al. (2016) for this study area, male cheetahs frequently use habitat margins, possibly due to increased visibility of prey in adjacent habitats and concealment during approach (Caro 1994; Muntifering et al. 2006; Nghikembua et al. 2016). Areas with intermediate cover, where bush encroachment is low to moderate, may provide sufficient habitat margins but also large tracts that are mostly unobstructed by shrubs, which could promote successful high-speed chases of prey (Myers 1975; Caro 1994; Mills et al. 2004). Intermediate cover may also allow cheetahs to consume prey out of direct sight of kleptoparasites (Mills et al. 2004), thereby increasing kill retention time. However, we expected a greater difference in FWC thresholds between cheetah and leopard. It is possible that cheetahs in our system have adapted to tolerate slightly more woody vegetation than in other parts of their range, and/or leopards make effective use of non-woody cover (e.g., grass) to ambush prey. We recommend that more studies examine predation strategies of large carnivores under variable FWC conditions and bush encroachment scenarios.
Our hypothesis (3) was rejected for both predator species. Higher inferred leopard predation success with increased distance from water may be the result of prey avoidance of waterpoints. While prey have been shown to gather at permanent waterpoints during the dry season (Thrash et al. 1995; Rispel and Lendelvo 2016), leopards may often use water points provided high woody cover is available (Maputla et al. 2015; CCF unpublished data) and this could have led to strong avoidance of waterholes by prey during leopard visits.
We found partial support for hypothesis (4) for cheetah, but not for leopard. Prey availability is generally higher on protected land within our study area (CCF 2021), which could partially explain the positive association between cheetah satiation and proportion of protected area (Fig. 3b). The pattern of high prey abundance in protected areas compared to unprotected land is common throughout Africa wherein parks and reserves are important for the protection of cheetahs (Cristescu et al. 2017) and other wildlife. Protected land is becoming increasingly isolated due to encroachment by human settlements, expansion of rangelands for livestock, and in some areas fencing, which poses challenges to the long-term conservation of an abundant and viable prey base for carnivores across many parts of Africa (Newmark 2008).
The lack of support for leopard wet season models may indicate that the assessed variables are not good predictors of leopard predation success in this season. This is possibly because precipitation occurring in the wet season promoted growth of sufficient foliage to camouflage leopards, thereby reducing the influence of FWC on predation success. Alternatively, the influence of FWC on leopard predation success may have been masked by high kleptoparasitism of kills in the wet season. To assess such effects, it would be necessary to monitor collared individual leopards and visit feeding sites rapidly after a suspected kill has occurred.
It is likely that the lack of predictive power of the cheetah dry season models resulted from the small sample size (n = 12), as cheetah preference for higher grass cover and decreased shrub height has been described for the study area (Muntifering et al. 2006; Nghikembua et al. 2016). Lack of data for this season may have occurred from shifts in cheetah home ranges away from the area during the dry season (Marker et al. 2008), leading to decreased detection. An association between cheetah predation success and FWC might be seen with a larger sample size.
Findings from this study suggest that managing bush cover at around 25–30% (Fig. 3a, c) in the region would help ensure high predation success of both cheetah and leopard. Potentially lower predation success in areas of very low or very high bush cover may ultimately affect individual and population fitness through impacts on growth and reproduction (Van Noordwijk and de Jong 1986).
Bush encroachment changes ecosystem structure, alters ecological communities, and affects predator–prey interactions (Hobbs and Mooney 1986; Oba et al. 2000; De Klerk 2004; Nghikembua et al. 2016; Soto-Shoender et al. 2018). It is likely to continue to impact savannas and grasslands at an accelerated rate due to increased atmospheric carbon and heightened grazing pressure on grasses when livestock are overstocked. Our analysis suggests that bush encroachment could affect predation success of apex predators and the thresholds identified herein could inform habitat management for the conservation of target species. Obtaining SAR-derived data for bush-encroached areas elsewhere to complement belly score assessments of top predators will be helpful in furthering understanding of the generality of these patterns across geographic regions.
Conclusions
This study highlights the importance of low to medium levels of woody plant species cover, and by extension habitat heterogeneity, for the predation success of two apex predators coexisting in a semi-arid savanna. Bush encroachment threatens to homogenise landscapes and remove habitats that enable large predators to hunt and consume prey, thereby affecting energetic trade-offs and predator–prey interactions. Objective, quantitative belly score methods provide an immediate indicator of food consumption for large carnivores and have the potential to serve as an early warning of future declines in fitness when used to index predation success for a given species and population. We encourage researchers to build on findings from this study and to trial the belly scoring approach herein for gaining non-invasive insights into predator–prey interactions and community ecology of apex predators in savanna and grassland study systems impacted by habitat change.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29(8):1123–1144
Balme GA, Hunter L, Slotow ROB (2007) Feeding habitat selection by hunting leopards Panthera pardus in a woodland savanna: prey catchability versus abundance. Anim Behav 74(3):589–598
Balme GA, Hunter L, Braczkowski AR (2012) Applicability of age-based hunting regulations for African leopards. PLoS ONE 7(4):e0035209
Balme GA, Batchelor A, de Woronin BN, Seymour G, Grover M, Hes L, Macdonald DW, Hunter LTB (2013) Reproductive success of female leopards Panthera pardus: the importance of top-down processes. Mamm Rev 43(3):221–237
Barnard P (ed) (1998) Overview of Namibia and its biological diversity. Biological Diversity in Namibia. Namibian National Biodiversity Task Force, Windhoek, Namibia, pp 15–55
Bertram BCR (1975) Weights and measures of lions. East Afr Wild Life Soc 13:141–143
Bissett C, Bernard RTF (2007) Habitat selection and feeding ecology of the cheetah (Acinonyx jubatus) in thicket vegetation: is the cheetah a savanna specialist? J Zool 271(3):310–317
Bjørneraas K, Herfindal I, Solberg EJ, Sæther BE, van Moorter B, Rolandsen CM (2012) Habitat quality influences population distribution, individual space use and functional responses in habitat selection by a large herbivore. Oecologia 168(1):231–243
Bond WJ, Midgley GF (2000) A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob Change Biol 6(8):865–869
Borstlap DG (2002) Intake and digestibility studies with captive African lions (Panthera leo), leopards (Panthera pardus) and cheetahs (Acinonyx jubatus). Doctoral dissertation, University of the Free State, South Africa
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Caro TM (1987) Cheetah mothers’ vigilance: looking out for prey or for predators? Behav Ecol Sociobiol 20:351–361
Caro TM (1994) Cheetahs of the Serengeti Plains: group living in an asocial species, 2nd edn. University of Chicago Press, Chicago
Chatterjee S, Hadi AS, Price B (2000) Regression analysis by example, 5th edn. Wiley, New York
Cheetah Conservation Fund (2021) Ecology department 30-year internal report. Cheetah Conservation Fund, Windhoek
Cloutier TL (2020) Anthropogenic Impacts and Influence on African Painted Dogs (Lycaon pictus). Doctoral dissertation, Antioch University New England, New Hampshire
Constant NL, Bell S, Hill RA (2015) The impacts, characterisation and management of human–leopard conflict in a multi-use land system in South Africa. Biodivers Conserv 24:2967–2989
Cornhill KL, Kerley GI (2020) Cheetah behaviour at scent-marking sites indicates differential use by sex and social rank. Ethology 126(10):976–986
Cristescu B, Lindsey P, Maes O, Bissett C, Mills G, Marker L (2017) Protected areas for cheetah conservation. In: Marker L, Schmidt-Küntzel A, Boast L, Nyhus P (eds) Cheetahs: biology and conservation. 1st Ed. Series: Biodiversity of the world: conservation from genes to landscapes. Elsevier, London, pp 265–274
Crowell MM, Shipley LA, Camp MJ, Rachlow JL, Forbey JS, Johnson TR (2016) Selection of food patches by sympatric herbivores in response to concealment and distance from a refuge. Ecol Evol 6(9):2865–2876
Davies AB, Tambling CJ, Kerley GI, Asner GP (2016) Effects of vegetation structure on the location of lion kill sites in African thicket. PLoS ONE 11(2):e0149098
De Klerk JN (2004) Bush encroachment in Namibia: Report on Phase 1 of the Bush Encroachment Research. Monitoring and Management Project, Ministry of Environment and Tourism, Windhoek, Namibia. https://dasnamibia.org/wp-content/uploads/2016/07/De-Klerk-Bush-Encoachment-in-Namibia-2004.pdf. Accessed 7 Feb 2022
Fabiano EC, Boast LK, Fuller AK, Sutherland C (2018) The use of remote camera trapping to study cheetahs: past reflections and future directions. In: Marker L, Schmidt-Küntzel A, Boast L, Nyhus P (eds) Cheetahs: biology and conservation. 1st Ed. Series: Biodiversity of the world: conservation from genes to landscapes, Elsevier, London, pp 415–425
Fabiano EC, Sutherland C, Fuller AK, Nghikembua M, Eizirik E, Marker L (2020) Trends in cheetah Acinonyx jubatus density in north-central Namibia. Popul Ecol 62(2):233–243
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks
GIMP Development Team (2021) GIMP. https://www.gimp.org. Accessed 2 Feb 2022
Gorini L, Linnell JD, May R, Panzacchi M, Boitani L, Odden M, Nilsen EB (2012) Habitat heterogeneity and mammalian predator–prey interactions. Mammal Rev 42(1):55–77
Gros PM, Rejmánek M (1999) Status and habitat preferences of Uganda cheetahs: an attempt to predict carnivore occurrence based on vegetation structure. Biodivers Conserv 8(11):1561–1583
Hayward MW, Henschel P, O’Brien J, Hofmeyr M, Balme G, Kerley GI (2006a) Prey preferences of the leopard (Panthera pardus). J Zool 270(2):298–313
Hayward MW, Hofmeyr M, O’Brien J, Kerley GI (2006b) Prey preferences of the cheetah (Acinonyx jubatus)(Felidae: Carnivora): morphological limitations or the need to capture rapidly consumable prey before kleptoparasites arrive? J Zool 270(4):615–627
Hayward MW, O’Brien J, Kerley GIH (2007) Carrying capacity of large African predators: predictions and tests. Biol Conserv 139:219–229
Hebblewhite M, Merrill EH, McDonald TL (2005) Spatial decomposition of predation risk using resource selection functions: an example in a wolf–elk predator–prey system. Oikos 111(1):101–111
Heithaus MR (2001) Habitat selection by predators and prey in communities with asymmetrical intraguild predation. Oikos 92(3):542–554
Hobbs RJ, Mooney HA (1986) Community changes following shrub invasion of grassland. Oecologia 70(4):508–513
Hoffman MT, Rohde RF, Gillson L (2019) Rethinking catastrophe? Historical trajectories and modelled future vegetation change in southern Africa. Anthropocene 25:100189
Houser AM, Somers MJ, Boast LK (2009) Home range use of free-ranging cheetah on farm and conservation land in Botswana. S Afr J Wildl Res 39(1):11–22
Hunter LTB, Hamman D (2003) Cheetah. Struik Publishers, Cape Town
Jeltsch F, Milton SJ, Dean WR, Van Rooyen N (1997) Analysing shrub encroachment in the southern Kalahari: a grid-based modelling approach. J Appl Ecol 1497–508
Kiffner C, Rheault H, Miller E, Scheetz T, Enriquez V, Swafford R, Kioko J, Prins HH (2017) Long-term population dynamics in a multi-species assemblage of large herbivores in East Africa. Ecosphere 8(12):e02027
Klaassen B, Broekhuis F (2018) Living on the edge: multiscale habitat selection by cheetahs in a human-wildlife landscape. Ecol Evol 8(15):7611–7623
Knobel DL, Du Toit JT, Bingham J (2002) Development of a bait and baiting system for delivery of oral rabies vaccine to free-ranging African wild dogs (Lycaon pictus). J Wildl Dis 38(2):352–362
Laundré JW, Hernández L, Altendorf KB (2001) Wolves, elk, and bison: reestablishing the" landscape of fear" in Yellowstone National Park, USA. Can J Zool 79(8):1401–1409
Le Roux E, Kerley GI, Cromsigt JP (2018) Megaherbivores modify trophic cascades triggered by fear of predation in an African savanna ecosystem. Curr Biol 28(15):2493–2499
Lindsey PA, Petracca LS, Funston PJ, Bauer H, Dickman A, Everatt K, Flyman M, Henschel P, Hinks AE, Kasiki S, Loveridge A (2017) The performance of African protected areas for lions and their prey. Biol Conserv 209:137–149
Loggins AA, Shrader AM, Monadjem A, McCleery RA (2019) Shrub cover homogenizes small mammals’ activity and perceived predation risk. Sci Rep 9(1):1–1
Maputla NW, Maruping NT, Chimimba CT, Ferreira SM (2015) Spatio-temporal separation between lions and leopards in the Kruger National Park and the Timbavati Private Nature Reserve, South Africa. Glob Ecol Conserv 3:693–706
Marker LL, Dickman AJ (2005) Factors affecting leopard (Panthera pardus) spatial ecology, with particular reference to Namibian farmlands. S Afr J Wildl Res 35(2):105–115
Marker LL, Dickman AJ, Mills MG, Jeo RM, Macdonald DW (2008) Spatial ecology of cheetahs on north-central Namibian farmlands. J Zool 274(3):226–238
Marker-Kraus L, Kraus D, Barnett D, Hurlbut S (1996) Cheetah survival on Namibian farmlands, 1st edn. Cheetah Conservation Fund, Windhoek
Martin J, Owen-Smith N (2016) Habitat selectivity influences the reactive responses of African ungulates to encounters with lions. Anim Behav 116:163–170
Martinussen MB, Båmstedt U (1999) Nutritional ecology of gelatinous planktonic predators. Digestion rate in relation to type and amount of prey. J Exp Mar Biol Ecol 232(1):61–84
Massawe AW, Mulungu LS, Makundi RH, Dlamini N, Eiseb SJ, Kirsten F, Mahlaba TA, Malebane P, Von Maltitz E, Monadjem A, Taylor P (2011) Spatial and temporal population dynamics of rodents in three geographically different regions in Africa: implication for ecologically-based rodent management. Afr Zool 46(2):393–405
Mendelsohn JM, Jarvis AM, Roberts CS, Robertson T (2002) Atlas of Namibia. Research and Information Services of Namibia, Namibia
Mills MGL, Broomhall LS, du Toit JT (2004) Cheetah Acinonyx jubatus feeding ecology in the Kruger National Park and a comparison across African savanna habitats: is the cheetah only a successful hunter on open grassland plains? Wildl Biol 10(1):177–186
Muntifering JR, Dickman AJ, Perlow LM, Hruska T, Ryan PG, Marker LL, Jeo RM (2006) Managing the matrix for large carnivores: a novel approach and perspective from cheetah (Acinonyx jubatus) habitat suitability modelling. Anim Conserv 9(1):103–112
Myers N (1975) The Cheetah Acinonyx jubatus in Africa—Report of a survey in Africa from the Sahara southwards. IUCN Monograph No. 4. IUCN, Morges, Switzerland
Newmark WD (2008) Isolation of African protected areas. Front Ecol Environ 6(6):321–328
Nghikembua MT, Harris J, Tregenza T, Marker LL (2016) Spatial and temporal habitat use by GPS collared male cheetahs in modified bushland habitat. Open J For 6(4):269–280
Nghikembua MT, Marker LL, Brewer B, Mehtätalo L, Appiah M, Pappinen A (2020) Response of wildlife to bush thinning on the north central freehold farmlands of Namibia. For Ecol Manag 473:118330
Nghikembua MT, Marker LL, Brewer B, Leinonen A, Mehtätalo L, Appiah M, Pappinen A (2021) Restoration thinning reduces bush encroachment on freehold farmlands in north-central Namibia. Forestry 94(4):551–564
Nowell K, Jackson P (1996) Wild cats: status survey and conservation action plan. IUCN/SSC Cat Specialist Group. IUCN, Gland, Switzerland
Oba G, Post E, Syvertsen PO, Stenseth NC (2000) Bush cover and range condition assessments in relation to landscape and grazing in southern Ethiopia. Landsc Ecol 15(6):535–546
O’Brien TG, Kinnaird MF, Wibisono HT (2003) Crouching tigers, hidden prey: sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6:131–139
O’Connor TG, Puttick JR, Hoffman MT (2014) Bush encroachment in southern Africa: changes and causes. Afr J Range Forage Sci 31(2):67–88
Ogutu JO, Owen-Smith N (2003) ENSO, rainfall and temperature influences on extreme population declines among African savanna ungulates. Ecol Lett 6:412–419
Potgieter KR, Davies-Mostert HT (2012) A simple visual estimation of food consumption in carnivores. PLoS ONE 7(5):e0034543
QGIS Development Team (2019) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. Accessed 2 Feb 2022
Riginos C, Grace JB (2008) Savanna tree density, herbivores, and the herbaceous community: bottom-up vs top-down effects. Ecology 89(8):2228–2238
Rispel M, Lendelvo S (2016) The utilization of water points by wildlife species in Nyae Nyae Conservancy, Namibia. Environ Nat Resour Res 6(4):91–103
Rostro-García S, Kamler JF, Hunter LTB (2015) To kill, stay or flee: the effects of lions and landscape factors on habitat and kill site selection of cheetahs in South Africa. PLoS ONE 10(2):e0117743
RStudio Team (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, USA. http://www.rstudio.com/. Accessed 2 Feb 2022
SAIEA (2016) Strategic environmental assessment of large-scale bush thinning and value-addition activities in Namibia: main report. Institute for Environmental Assessment, Windhoek
Scheiter S, Higgins SI (2009) Impacts of climate change on the vegetation of Africa: an adaptive dynamic vegetation modelling approach. Glob Change Biol 15(9):2224–2246
Schiffmann C, Clauss M, Hoby S, Hatt J-M (2017) Visual body condition scoring in zoo animals—composite, algorithm and overview approaches in captive Asian and African elephants. J Zoo Aquar Res 5:1–10
Singh JS, Joshi MC (1979) Ecology of the semi-arid regions of India with emphasis on land-use. In: Developments in agricultural and managed forest ecology, vol 7. Elsevier, pp 243–275
Soto-Shoender JR, McCleery RA, Monadjem A, Gwinn DC (2018) The importance of grass cover for mammalian diversity and habitat associations in a bush encroached savanna. Biol Conserv 221:127–136
Stein AB, Hayssen V (2013) Panthera pardus (Carnivora: Felidae). Mamm Species 45(900):30–48
Stein AB, Fuller TK, DeStefano S, Marker LL (2011) Leopard population and home range estimates in north-central Namibia. Afr J Ecol 49(3):383–387
Sunquist M, Sunquist F (2002) Wild cats of the world. University of Chicago Press, Chicago
Thrash I, Theron GK, Bothma JDP (1995) Dry season herbivore densities around drinking troughs in the Kruger National Park. J Arid Environ 29(2):213–219
Valeix M, Loveridge AJ, Davidson Z, Madzikanda H, Fritz H, Macdonald DW (2010) How key habitat features influence large terrestrial carnivore movements: waterholes and African lions in a semi-arid savanna of north-western Zimbabwe. Landsc Ecol 25:337–351
Van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128(1):137–142
Verschueren S, Briers-Louw WD, Cristescu B, Fabiano E, Nghikembua M, Torres-Uribe C, Walker EH, Marker L (2021) Spatiotemporal sharing and partitioning of scent-marking sites by cheetahs and leopards in north-central Namibia. Afr J Ecol 59:605–613
Walker EH, Nghikembua M, Bibles B, Marker L (2016) Scent-post preference of free-ranging Namibian cheetahs. Glob Ecol Conserv 8:55–57
Walter H (1964) Productivity of vegetation in arid countries, the savannah problem and bush encroachment after overgrazing. Ecol Man Trop Environ 4:221–229
Wessels K, Mathieu R, Knox N, Main R, Naidoo L, Steenkamp K (2019) Mapping and monitoring fractional woody vegetation cover in the arid savannas of Namibia using LiDAR training data, machine learning, and ALOS PALSAR data. Remote Sens 11(22):2633
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Winterbach HEK, Winterbach CW, Somers MJ, Hayward MW (2013) Key factors and related principles in the conservation of large African carnivores. Mamm Rev 43(2):89–110
Acknowledgements
We thank Dr. Konrad Wessels (George Mason University) for providing the fractional woody cover GIS layer. This dataset was derived by Dr. Konrad Wessels from products produced by the Council of Scientific and Industrial Research (CSIR), South Africa, with their permission. Airborne LiDAR data used to derive the products were provided by Southern Mapping Company, South Africa, with permission from NamPower (Namibia). We thank Tammy Cloutier (Antioch University of New England) for feedback on the belly score method, which formed the basis of the camera trap data analysis. Funding for this research was provided by the Cheetah Conservation Fund and its donors. Stijn Verschueren (Cheetah Conservation Fund) compiled the camera trap data used for this research.
Funding
This work was supported by the Cheetah Conservation Fund and its donors.
Author information
Authors and Affiliations
Contributions
HA, BC and NR formulated the study idea, HA and BC developed the methodology, LM collected the data, BC and LM provided access to data, HA selected data for analysis, analysed the data and drafted the manuscript, BC and NR provided academic and editorial advice, LM, NR, BC provided resources and reviewed the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Ethics approval for this research was waived by the University of Bristol Animal Welfare and Ethical Review Body (reference number: UIN/21/026).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atkinson, H., Cristescu, B., Marker, L. et al. Habitat thresholds for successful predation under landscape change. Landsc Ecol 37, 2847–2860 (2022). https://doi.org/10.1007/s10980-022-01512-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01512-x