Abstract
Context
The agricultural landscape in many low- and middle-income countries is characterized by smallholder management systems, often dependent on ecosystem services, such as pollination by wild pollinator populations. Increased adoption of modern inputs (e.g., agrochemicals) may threaten pollinators and smallholder crop production.
Objective
We aimed to identify the link between the use of agrochemicals and wild bee populations in Southern India, while explicitly considering the effects of temporal and spatial scaling.
Methods
For our empirical analysis, we combined data from pan trap samples and a farm management survey of 127 agricultural plots around Bangalore, India. We implemented a Poisson generalized linear model to analyze factors that influence bee abundance and richness with a particular focus on the present, past, and neighboring management decisions of farmers with respect to chemical fertilizers, pesticides, and irrigation.
Results
Our results suggest that agricultural intensification is associated with a decrease in the abundance and richness of wild bees in our study areas. Both time and space play an important role in explaining farm-bee interactions. We find statistically significant negative spillovers from pesticide use. Smallholders’ use of chemical fertilizers and irrigation on their own plots significantly decreases the abundance of bees. Intensive past management reduces both bee abundance and richness.
Conclusions
Our results suggest that cooperative behavior among farmers and/or the regulation of agrochemical use is crucial to moderate spatial spillovers of farm management decisions. Furthermore, a rotation of extensive and intensive management could mitigate negative effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollinator communities make important contributions to agricultural production and food security (Tscharntke et al. 2012; Kleijn et al. 2015). With the fast decline of pollinator populations both globally (Tylianakis 2013) and regionally, for example, in India (Basu et al. 2011), interest in this topic has increased substantially in recent years. While many staple crops do not rely on animal pollination, most fruit and vegetable crops do (Klein et al. 2007). These crops often feature prominently in efforts to improve the incomes and living standards of smallholders in low-income countries. Commercialization of high-value fruit and vegetable production systems allows participation in national and international agricultural value chains and, therefore, can contribute to economic development (Maertens et al. 2012). As a consequence, agricultural policy strategies in many low-income countries focus on improving farmers’ access to modern inputs and technologies to encourage more commercialized agricultural practices (Minten et al. 2013; Jayne et al. 2018). Furthermore, improved infrastructure and better access to urban centers and markets are also accelerating the transformation from extensive to more intensified agricultural management systems in large parts of Africa and Asia (Vandercasteelen et al. 2018; Steinhübel and von Cramon-Taubadel 2021).
Despite the need for intensification to improve yields and foster economic development, greater use of modern agricultural technologies and agrochemicals such as pesticides can harm pollinator populations, with negative implications for future economic performance (Brittain et al. 2010; Goulson et al. 2015). Evidence on farm-pollinator interactions in higher-income countries is vast, but there are only a handful of studies on rural–urban landscapes in lower-income countries and tropical regions (Wenzel et al. 2020). Some notable and recent exceptions are the studies by Basu et al. (2016), Chakraborty et al. (2021), and Tommasi et al. (2021), who investigate farm/landscape-pollinator interactions in India and northern Tanzania. Despite these important contributions, more empirical evidence on the interactions between landscape structure and pollinator communities in tropical countries is needed. Farming systems can differ greatly between high- and low-income countries, and we cannot simply extrapolate from the existing literature to fully understand farm-pollinator interactions in low-income countries or tropical localities. For example, agricultural plots in low-income countries are often much smaller, and climate and crop choices differ (Basu et al. 2016). Also, smallholders usually depend exclusively on wild pollinator populations (Tibesigwa et al. 2019). When wild populations cannot provide sufficient pollination services, farmers in high-income countries often bring in managed bee populations as a substitute, but this option is often not available or affordable for smallholders in low- and middle-income countries (Tibesigwa et al. 2019). Hence, the first motivation for this study is to provide further evidence of farm-pollinator interactions based on a sample of 127 smallholder farms located around Bangalore in Southern India. The region is dominated by smallholder agriculture, but the proximity to the urban center of Bangalore provides a multitude of marketing options to farmers and access to modern technologies and inputs such as agrochemicals. Consequently, management systems have become increasingly intensified, making the region a prime example of the issues outlined above.
The second motivation of our study is to present an empirical strategy that combines data from pan trap experiments and household surveys to quantify spatial and temporal spillovers in the effects of local farm management decisions on pollinator communities.Footnote 1 Here, scale dependencies play a critical role. Several ecological studies demonstrate the importance of landscape scale, structure, composition, and configuration in defining pollination services to account for pollinator mobility and foraging ranges (Tscharntke et al. 2005; Kremen et al. 2007; Halinski et al. 2020). As a consequence, farm management is often considered in an aggregate fashion and non-crop plants or access to natural habitats (e.g., home gardens versus natural forests) play a major role when analyzing pollinator communities (Tscharntke and Brandl 2004; Tscharntke et al. 2005; Blitzer et al. 2012; Motzke et al. 2016; Tibesigwa et al. 2019; Viswanathan et al. 2020). However, since pollinators can move between agricultural plots and natural habitats, ecological and anthropogenic boundaries do not necessarily coincide.
Only recently have ecological studies started to look into scale-dependencies of farm-pollinator interactions by contrasting the effects of local and landscape land-use features on pollinator diversity (Basu et al. 2016; Chakraborty et al. 2021; Tommasi et al. 2021). Nonetheless, despite the explicit consideration of local factors, except for Tommasi et al. (2021), these studies still chose their sampling sites based on landscape and regional characteristics (e.g., overall agricultural input intensities or cultivation patterns) rather than human-defined boundaries such as agricultural plots (Basu et al. 2016). Such boundaries are, however, relevant from a policy perspective, since landscape-level or regional patterns are of limited use when trying to understand the effects of farmers’ decisions, which are typically made at the household or farm level.
Similarly, farm management is often associated with short-term seasons or growing cycles (Steinhübel et al. 2021), while ecological studies emphasize that pollinator communities are likely affected by the longer-term accumulation of management decisions that affect nesting and foraging possibilities or exposure to pollutants and toxicants (Kremen et al. 2007; Chakrabarti et al. 2015; Schwarz et al. 2020).
In this context, our aim is to answer three research questions by specifically modeling the effect of present, past, and neighboring agricultural management choices on bee abundance and richness.
-
(1)
What are the effects of different agricultural inputs (specifically, chemical fertilizer, pesticides, and irrigation) on bee communities?
-
(2)
How do farm management practices on one farm affect bee communities on other farmers’ plots, i.e., to what extent do spillover effects of farm management extend beyond the boundaries of management units (spatial spillover)?
-
(3)
How does past management affect current bee communities (temporal spillover)?
By considering different scales and looking at the use of different agricultural inputs, our results contribute to a more detailed understanding of farm-pollinator interactions. This can help to improve the targeting of extension and policy measures to regulate the use of agricultural inputs that can harm bee communities, and therefore ultimately support sustainable agricultural growth in low-income countries.
Methods
Study areas and survey design
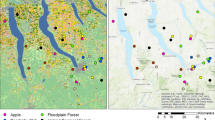
Our empirical analysis is based on data from two study areas that extend from urban Bangalore roughly 40 km into the surrounding rural–urban interface, one to the north and the other to the south and west (Fig. 1). The rural–urban interface is heavily influenced by the rapidly growing city of Bangalore; the last official census in 2011 recorded 9.6 million inhabitants and average annual growth rates of about 8% (Directorate of Census Operations Karnataka 2011). The rural–urban interface surrounding Bangalore is dominated by smallholder agriculture and agricultural land use is small in scale and highly fragmented. Bangalore and several satellite towns offer a variety of marketing opportunities to farmers and connect them to regional, national, and even international markets. Expanding infrastructure also improves farmers’ access to input markets, especially for chemical fertilizers and pesticides. Consequently, agricultural production is becoming increasingly commercial, and many farmers are shifting from subsistence production of staple crops to intensive fruit and vegetable production. The agricultural production systems in the rural–urban interface of Bangalore thus exemplify the dilemma that we discuss in the introduction: smallholders are shifting to more pollinator-dependent production systems and simultaneously increasing the use of potentially pollinator-harming inputs.
Two research areas to the north and south-west of Bangalore displaying the location of plots sampled along the rural–urban interface of Bangalore, Southern India (\(N=127\)). Panels on the right show zoomed-in representations of the grey-shaded squares in the large map. The village and plot coordinates were collected during the household survey in 2018. All other map features were downloaded from OpenStreetMap and visualized with QGIS
To capture the potential spatial heterogeneity induced by the urban center of Bangalore, the selection of farm households and plots for data collection followed a two-step approach. Based on the Survey Stratification Index (SSI) introduced by Hoffmann et al. (2017), all villages in the two study areas were classified into three strata (rural, peri-urban, and urban) based on their distance from Bangalore and the village built-up area (1 km radius) calculated based on satellite images. In the first step, we randomly selected ten villages in each stratum in each study area (60 villages in total), and randomly drew an average of 20 households per village (weighted by village size) from household lists provided by preschool teachers in the selected villages. Between December 2016 and May 2017, the resulting 1275 households were subjected to a detailed baseline socioeconomic survey. About half of these households (638) were farm households; that is, they managed at least one plot in 2016. For these households, the baseline survey included data on agricultural management in the agricultural year 2016/2017 and recall data for the years 2012 to 2015.
In the second step, we drew a random subsample of 24 villages: 12 of the 20 in the peri-urban and 12 of the 20 in the rural stratum.Footnote 2 In these randomly selected villages, all households that had been identified as farm households in the first step were selected for pan trap experiments (described below) and a second farm management survey, which was carried out in February and March 2018.Footnote 3 This second survey covered information on agricultural management decisions in the 2017/18 season. The combination of data from both surveys provided us with a continuous record of the management history of each of the 127 farm households extending back to 2012. (An overview of sample demographic characteristics and knowledge about pollination can be found in Online Tables A.1, A2).
We used pan traps to sample pollinators, a standard rapid sampling method to record pollinator communities (Westphal et al. 2008; Meyer et al. 2015). We randomly selected one plot from each of the 127 farm households in the subsample (Fig. 1). We collected information on the direct neighborhood of each selected plot, as well as the GPS coordinates of its centroid. Four pan traps were placed on each of these plots near the field margins. The pan traps were 500 ml bowls sprayed with yellow UV-bright color and filled with unscented soapy water to break the surface tension. To ensure that we captured as many pollinators as possible, all four pan traps were placed near flower-rich patches, with a minimum distance of approximately 10 m between traps to minimize interactions between them. The traps were collected after 48 h of exposure. Unfortunately, some traps failed; they spilled or were taken away by passers-by. As a consequence, some plots had fewer than four successful pan traps; we introduced dummy variables in our later analysis to control for the number of successful traps per plot (see Online Table A.3). Pan traps were placed in the field on dry, bright, and mostly calm days between 10 am and 2 pm. The collection took place from January 9 to February 11, 2018.
After collection, all insects caught in the traps were treated with 70% ethanol, pinned, and identified to species or genus level. Most of the captured insects were bees along with a few other pollinating insect taxa (e.g., beetles, butterflies, flies, and wasps). Since pollinator groups differ greatly in their ecological characteristics (Gagic et al. 2015), we decided to consider only bees in our analysis. In the remainder of this article, 'species' refers to the lowest taxonomic rank identified. To our knowledge, beekeeping is not common in the study areas. None of our sampled households reported keeping bees. Therefore, we assume that most of the bees caught in the pan traps originate from wild populations.
We used the number of bees caught per plot as a proxy for bee abundance and the number of different bee species as a proxy for bee richness. We are aware that these are only rough indicators of local bee diversity and that pan traps might oversample smaller species (see e.g., Baum and Wallen 2011). Nevertheless, both indicators are frequently used in the ecological literature ( Kremen et al. 2002, 2004; Holzschuh et al. 2007) and hence our results can easily be compared with previous studies. Since the abundance and richness of bees are highly correlated in our sample (\(\rho =0.919\)), we do not use both in the same model specification to avoid multicollinearity problems.
Empirical analysis
We implemented a Poisson generalized linear model (GLM) with two dependent variables: plot-level abundance and richness counts.Footnote 4 Since our main interest is in investigating how these counts are affected by farm management decisions, we define three key variables for our analysis, namely the use of chemical fertilizers, irrigation, and pesticides. Chemical fertilizers and irrigation are commonly used to quantify the intensity of agricultural management in low- and middle-income countries (see e.g., Asfaw et al. 2016; Vandercasteelen et al. 2018). For ground-nesting bees, for example, irrigation can also have some more direct effects (e.g., Esther Julier and Roulston 2009) and thus captures an important additional dimension of potential farm-pollinator interactions. Pesticides not only signal intensification but can reduce foraging resources (e.g., in the case of herbicide applications) or be even directly harmful to bees (Brittain et al. 2010; Tuell and Isaacs 2010). The three agricultural input variables in our analysis were based on reported input use by household agricultural decision makers, where the chemical fertilizer category comprises all inorganic fertilizers and irrigation refers to any irrigation source apart from rain (e.g., borewell). The pesticide category includes all reported use of insecticides, herbicides, and fungicides.
For the years 2017 and 2018, we have data on the reported amounts of fertilizer and pesticide applied on the sample plots. Nonetheless, we refrained from including input quantities as explanatory variables because we also wanted to include information on past plot management decisions in our analysis, and for earlier years (2012 to 2016) we only have data on whether a given input was used, not quantities.Footnote 5 Therefore, all three agricultural input variables are binary variables, where a value of 1 indicates that the farmer applied the respective input in the year in question, and 0 otherwise.
To incorporate the different temporal and spatial scales discussed before, we estimated the effects of current farm practices (chemical fertilizer, irrigation, pesticides) on the plot under observation (present management) but also allowed for the possible effects of past farm practices on the same plot (past management) as well as farm practices on plots in the neighborhood (neighboring management). We define past management as the number of years in which a farm practice was used on the plot under observation since 2012. Neighboring management is the share of smallholders in our sample in a radius of 2 or 4 km around the plot under observation applying a farm practice (Table 1; for a detailed and more technical description of the model and construction of the variables of neighboring management, see Online Appendix 1).
To ensure that we capture the actual effect of farm management on bee abundance and richness, we consider 25 additional variables at the landscape and local scale (for a list and descriptive statistics, see Online Table A.3) to control for other potential influences on bee communities. Since our sample comprises only 127 observations, including all 25 variables would likely lead to overparameterization of the model. Therefore, we applied an adaptive selection algorithm based on the improved Akaike information criterion (iAIC), which evaluates the contribution of every variable to the model fit. Variables that do not improve the model were dropped (for details, see Belitz and Lang 2008; Umlauf et al. 2015).
At the landscape scale, the controls are the distance from Bangalore city center to control for exogenous spatial heterogeneity induced by the rural–urban gradient, a dummy for the southern study area to capture any study area-specific effects, and the built-up area within a 1 km radius of the village center to measure localized urbanization (for details, see Hoffmann et al. 2017).
At the plot level, we asked all farmers in the survey whether the plot with the pan traps has a common border with another agricultural plot, a fallow plot, a forest, a building, a road, or a body of water (e.g., a lake or river). We then used this information to create dummy variables that capture the adjacent land use of each plot. Furthermore, we included several variables that are related to pan traps and their placement and could therefore influence the abundance of bees. These variables are the number of successful pan traps per plot and meteorological variables such as cloud cover, temperature, and wind conditions when the pan traps were in place.
Since the cropping systems in the Bangalore area are diverse, we also controlled for different crops. In the 127 pan trap plots, 40 different crops were grown. This diversity of crops presents two main challenges. First, different crops serve bee communities in different ways and certain management practices might be strongly correlated with certain crops. Second, different crops have different growing schedules. As a consequence, some plots had already been harvested when the pan traps were placed, while others were in various earlier stages of development. Cropping seasons have become even more fluid with the increasing availability of irrigation, and there is no time of year when all agricultural plots are in a comparable state. We used different variables to test and control for these potential confounding effects. We introduced a categorical variable that indicates whether the plot was already harvested or had been fallow for the season. In addition, we controlled for functional groups of crops, namely flowers, fruits, staples, tree crops, and vegetables on the plots (see Online Table A.5 for detailed information). We refrained from adding crop-specific dummies because given 40 different crops this would have severely reduced the degrees of freedom for estimation. We also created a dummy variable indicating whether a crop is classified as a bee forage crop (i.e., pollen or nectar source); this variable represents the forage quality of the plot in the current season. Furthermore, we used the recall data from the baseline survey to measure the number of years since 2012 in which a plot had been planted with bee forage crops. Finally, we estimated the floral abundance in the focal crop on the plot when the pan traps were in place and the number of flowering plant (crop and non-crop) species within a 2 m radius of the pan traps.
Results
Overall, we caught 613 individual bees and identified 31 species belonging to three different families (Apidaea, Halictidae and Megachilidae, Online Table A.6). The most abundant species were Apis florea, Lasioglossum sp. 1, and Apis cerana (160, 83, and 79 individuals, respectively). The Chao 1 species richness estimators (Chao 1984) indicate that we sampled 88% of the regional bee species pool, and the species accumulation curve in Online Fig. 2 confirms that our sampling effort was sufficient to detect most bee species in the study areas.
Table 2 presents the results including all variables identified by the selection algorithm. To facilitate interpretation, we report marginal effects calculated at the mean rate of bee abundance and richness (instead of reporting coefficient estimates).
Effects of agricultural input use on bee communities
Our results provide evidence of a negative association between agricultural intensification and bee communities in the rural–urban interface of Bangalore. If a farmer applies chemical fertilizers or irrigation on a plot, this decreases bee abundance by about 20%. In terms of neighbor management, we found that the use of pesticides by other smallholders within 2 km of a plot reduces the abundance of bees in that plot. With every additional percent of pesticide use in the neighborhood of a plot, the number of bees on that plot decreases by one percent. Considering that on average 25% (maximum of 80%) of neighboring farmers apply pesticides (Table 1), the use of pesticides by neighboring smallholders can affect the abundance of bees on a plot as strongly as the use of intensive inputs on the plot itself. Regarding past management, we find that each additional year of irrigation, is associated with a decrease of the abundance of bees on a plot by 8.1%. Originally, the selection algorithm suggested including both past irrigation and pesticide use. However, since past irrigation and past pesticide use are significantly correlated (\(\rho =0.405\) (\(p\) < 0.001)), we decided to drop one of them to avoid multicollinearity.Footnote 6 Consequently, the effect of past irrigation should be interpreted as a general effect of past intensive agricultural management. The results for the relationship between agricultural management and bee richness are similar to those for bee abundance (Table 2). However, neither the present use of chemical fertilizers and irrigation, nor the neighboring use of pesticides, have statistically significant effects on bee richness. Past agricultural management, however, does have a significant negative effect on bee richness that is of roughly the same magnitude as its effect on bee abundance.
Other factors influencing bee communities
The selection algorithm only indicates two control variables that are positively associated with both bee abundance and richness. These are the categorical variable 'state of the plot' that controls for whether a plot was fallow or already harvested at the time of the pan trap experiment, and the number of flowering plant species around the pan traps. Both show relatively large effect sizes and high statistical significance. This finding is particularly important in light of the potential problem caused by the high crop diversity discussed in Sect. Empirical analysis. For example, the dummy variable 'plot already harvested' is negatively correlated with the irrigation dummy [\(\rho =-0.24\) (\(p\) = 0.008)]. Therefore, one might suspect that what appears to be an effect of irrigation on bee diversity might actually be due to which crop was grown and when it was harvested. However, note that when indicators of agricultural input use and control variables for crop choice are included in the same model, they produce relatively small standard errors and thus statistically significant estimates (Table 2).Footnote 7 Hence, we conclude that multicollinearity does not present a problem and we observe the actual effects of agricultural input use on bee diversity. This is further supported by variance inflation factors, which are smaller than 2.2 for all variables in Table 2.
Furthermore, the results in Table 2 show that a larger number of explanatory variables have statistically significant effects on bee abundance than on richness. This includes a statistically significant effect of the number of successful pan traps per plot, implying that this is an important control variable in the model for bee abundance. In addition, the use of the adjacent plot (i.e., roads, forest, water) appears to have a large influence on the abundance of bees. A road reduces bee abundance by 17.3%, whereas a neighboring forest or water body is associated with an increase of 30 and 39%, respectively.
On the landscape scale, the distance to the urban center of Bangalore appears to be an important factor in determining the number of bee species present. With every additional kilometer distance from the city, the bee richness increases by 2.4%. In contrast, the abundance of bees is negatively associated with the village built-up area, which is also an indicator of urbanization.
Discussion
The abundance and richness of bees or pollinators if often associated with pollination services (Cohen et al. 2021). Since we find that fertilizer, irrigation and pesticide use have negative effects on bee communities, it is reasonable to expect that they will also have negative effects on biodiversity and the ecosystem services such as pollination that are provided by bees. This negative relationship has been highlighted in the literature (Matson et al. 1997; Tilman et al. 2002; Winfree et al. 2009) and is confirmed in a recent study by Wenzel et al. (2022) for the crop Lablab (Lablab purpureus) in our study areas. However, since we did not measure direct pollination outcomes such as fruit or seed set, we cannot draw any conclusions about the effects of farm management practices on such outcomes; we can only conjecture based on other studies.
We do find that the abundance of bees in our study areas is negatively affected by spatial spillovers from neighboring smallholder management decisions, and especially by pesticide use. Several studies have analyzed the effect of pesticides on bee communities, but the results are not consistent. Whereas Tuell and Isaacs (2010), Park et al. (2015), and Basu et al. (2016) find significant negative effects of insecticides and pesticides, Kremen et al. (2004) and Shuler et al. (2005) do not find any effects of insecticide and overall pesticide use on wild pollinator populations, respectively. However, these studies (except for Basu et al. (2016)) do not consider spatial scaling and landscape-wide spillovers, which seems important in light of our estimation results. Studies that analyze effects at the landscape level typically only consider the effects of aggregated farming systems and land use in the landscape in question (for example measured in percentage shares of different types of land use), or the influence of distance to natural habitats (Holzschuh et al. 2007; Motzke et al. 2016; Nicholson et al. 2017; Tibesigwa et al. 2019; Viswanathan et al. 2020). A key advantage of our modeling approach is that it can identify spatial spillovers of specific plot-level farming practices, such as agricultural input use. This enables us to quantify the link between a farmer’s decision to use pesticides on a plot and the resulting externalities on other plots embedded within the same landscape.
Even if individual farmers were to reduce their use of pesticides locally to protect pollinator populations and services, they might still face decreased provision of pollination services due to continued use of pesticides by neighbors in the surrounding landscape. Regardless of which effect is more damaging—reduced yields and/or quality due to pests, or reduced yields due to a lack of pollinators (Catarino et al. 2019)—these farmers may find themselves in a prisoners’ dilemma situation. That is, due to a lack of coordination, each individual farmer loses more than if all farmers had cooperated in protecting pollinator populations on a larger spatial scale. At the other extreme, a free-riding problem might arise. If only one farmer applies pesticides while all others refrain to protect pollinators, then this farmer will likely benefit from lower pest rates, and from largely intact pollination services that would spill in from the surrounding landscape. Therefore, our results suggest that cooperative behavior among smallholders or other approaches, such as pesticide regulations that target a wider landscape scale, may be necessary to guarantee pollination services for all farmers. This is in line with other ecological studies (Goldman et al. 2007; Stallman 2011; Basu et al. 2016) that refer to the prisoners’ dilemma affecting pollinator maintenance (Rapoport 1989).
While in the global north intensified agriculture often takes place on large fields, in the global south agriculture is dominated by smallholders. Hence, issues arising from management spillovers might be even more relevant in the global south. In our study, the average plot size is about 0.54 hectares (Online Table A.3). This means that bee populations in Bangalore are more likely to be affected by neighboring management decisions than bee populations in Europe or North America. Often fragmented or diverse agricultural landscapes are associated with positive effects on pollinator populations (Krishnan et al. 2012; Chakraborty et al. 2021). In such landscapes pollinators benefit from forest patches, field margins, and other natural habitats between agricultural plots (Priess et al. 2007; Tibesigwa et al. 2019; Halinski et al. 2020; Viswanathan et al. 2020). We also find evidence of such a positive association. However, in landscapes dominated by small-scale agriculture, the local presence of pollinators also depends to a greater extent on the management decisions of neighbors. The question is then how to promote (cooperative) smallholder behavior towards more sustainable agricultural intensification. Elisante et al. (2019), for example, show in a case study in Tanzania that knowledge gaps play an important role and training can significantly increase sustainable management practices. Since many farmers in our study areas appear to be uncertain about the relationship between pollination and crop production (see Online Table A.2), such training might be helpful in this setting as well. Participatory programs, which involve farmers in monitoring activities, might be another option to achieve more sustainable and cooperative management strategies (Smith et al. 2017).
Unlike our results for pesticides, the negative effects of chemical fertilizers and irrigation on bee populations are limited to the plot level and do not appear to spill over between neighboring plots. Intensively managed plots likely offer fewer forage and nesting opportunities for bee populations than extensively managed plots, since they offer less natural vegetation. Furthermore, an increase in soil nitrogen due to intensive fertilization has been shown to significantly alter the composition of plant communities, as well as the phenology, morphology and production of nectar and pollen from plants (Ramos et al. 2018; David et al. 2019), all of which are critical factors in the determination of wild bee populations. This explains the local negative effect of chemical fertilizer and past intensive plot management on bee populations. Furthermore, several authors have emphasized the importance of time in determining pollinator access to species-specific forage and nesting resources (Kremen et al. 2007; Tuell and Isaacs 2010). However, note that farmers applied chemical fertilizers on 78% of the plots in our sample. Therefore, agriculture in our study areas is already quite intensive and, consequently there might be insufficient spatial variation of chemical fertilizer use in our sample to detect spatial spillovers.
Regarding other factors that influence bee abundance and richness, our results suggest that the distance to the urban center of Bangalore affects the number of bee species present, whereas abundance of bees is influenced by village built-up area. Urbanization does not follow a monotonic rural–urban gradient surrounding Bangalore; several smaller satellite towns influence urbanization patterns as well (Steinhübel and von Cramon-Taubadel 2021). In the vicinity of such towns, the built-up area and its negative effects can increase, even as one moves away from the Bangalore center. These findings match previous literature on the link between urbanization and pollinator decline (Wenzel et al. 2020; Tommasi et al. 2021). Physical infrastructure can impede biodiversity and ecosystem services due to changes in physical parameters (e.g., temperature), reduction of habitat size and connectivity (Faeth et al. 2011; Pickett et al. 2011; Turrini and Knop 2015), or light pollution (Altermatt and Ebert 2016). Furthermore, Banaszak-Cibicka and Zmihorski (2012) and Wenzel et al. (2022), for example, show that ground-nesting pollinators have bigger problems with urbanization than cavity-nesting species. This could be a sign that bee richness is more affected by landscape configuration on large scale, whereas bee abundance is influenced by more local factors. Further evidence of such a local influence on bee abundance is provided by the statistically significant effects of neighboring roads, bodies of water, and forests. Hence, our results provide evidence for the Bangalore area of the positive relationship between pollinator communities and forests or agroforestry systems that is emphasized in the literature (Motzke et al. 2016; Staton et al. 2019; Tibesigwa et al. 2019; Viswanathan et al. 2020).
Finally, the other statistically significant control variables show that the abundance of bees, and to a lesser extent the richness of bees, is subject to many influences. Among these, we find that the number of flowering crop and non-crop plant species and the number of successful pan traps have relatively large and statistically significant effect sizes. It is not surprising that we find evidence of significant effects of flowering crop and non-crop species, as bees feed on flowers and these effects are well established in the literature (e.g., Motzke et al. 2016; Laha et al. 2020; Tommasi et al. 2021). Furthermore, it is to be expected that measured bee abundance on a plot will depend on the number of successful traps. Our results confirm that a dummy variable for the number of successful traps should always be included in studies that face similar constraints.
Policy implications
Our results suggest that strategies to protect wild bee communities could include the regulation of pesticide use, but also the provision of incentives for cooperative behavior among farmers to coordinate their plot-level decisions and foster landscape-level improvements in pollinator habitats. This is particularly important in smallholder land-use systems, where plot sizes are relatively small and pollinator populations are affected by a multitude of interacting individual management decisions. Participatory approaches involving indigenous and local knowledge can contribute to such efforts. Extension services that increase farmers’ understanding of the importance of pollinators and how to protect them could also contribute. Trade-offs between pesticide use and pollination requirements have to be specifically addressed in such programs, as farmers will be more likely to support pollinator conservation if it leads to yield improvements.
Our results also show that a reduction in intensive input use on plots is associated with increased bee abundance. Since past plot management decisions affect current bee abundance and richness, rotating intensive and extensive management practices over time could help maintain sufficient forage and nesting opportunities to support healthy and diverse bee communities. Additionally, protecting forest patches or agroforestry plots also has the potential to promote bee populations.
Conclusions
The objective of this study is to evaluate the effects of agricultural management practices on bee communities and to add to the still scarce literature on farm-pollinator interactions in low-income countries based on primary data from the rural–urban interface of Bangalore. Studies are lacking particularly for countries with smallholder management systems. In our empirical analysis, we considered ecological factors on both the landscape and the plot scale, as well as farmers’ decisions to use different agricultural inputs on the plot scale. To account for spatial and temporal scaling, we applied a model that allows for spatial spillovers and the effects of past plot management.
Overall, we find statistically significant negative effects of agricultural intensification on the bee population in the Bangalore area. However, there are some differences between abundance and richness. While bee abundance is negatively affected by present, past, and neighboring farm management, bee richness only shows significant interactions with past farm management. We also find a statistically significant effect of the rural–urban gradient on bee richness, suggesting that landscape-level patterns also play an important role. In contrast, we find that the built-up area in the vicinity of a plot is negatively associated with bee abundance. These findings highlight the importance of considering spatial and temporal dimensions when analyzing farm-pollinator interactions.
To increase the statistical validity and precision of these results as a basis for policy recommendations, we need more and larger samples from different countries in the global south, ideally with several sampling periods of pollinator diversity. Data from other regions with fewer cultivated crops might reduce the correlation among variables and allow for more specific conclusions on the effects of different agricultural practices. Finally, to improve our understanding of economic implications and inform the design of effective policies, we also require research on the relationships between bee abundance and richness on the one hand, and pollination outcomes such as fruit set on the other.
Data availability
The data sets collected and analyzed during the current study are available from the corresponding author on reasonable request.
Notes
When we use the term ‘local’, we generally refer to the plot level. ‘Landscape’ we use to describe the spatial scale beyond the plot level containing several units of land use and natural habitats.
Because very few agricultural households are located in the urban stratum, we excluded the 20 villages in the urban stratum from the subsample.
Robust inference on spatial spillovers among farm plots requires enough observations (plots) within a potential interaction radius of one another. We therefore drew a random subsample of villages rather than households, to ensure that the individual observations (plots) are spatially clustered. See the zoomed-in areas in Fig. 1. However, in four of the villages in the peri-urban strata of the southern study area there was only one farm household. These households and several other households that are remote from the others in their respective villages were not considered in our empirical analysis. In the subsequent analysis we therefore consider 127 of the 144 households for which we have data on pollinator diversity and farm management.
All pan trap catches from a given plot were combined in the field for easier logistics. Therefore, we are unable to control for overdispersion in the empirical analysis. Our smallest unit of observation is the plot, not the individual pan trap.
We did not collect data on the quantities of inputs used in past years because the surveyed producers rarely keep records and their recollection of the quantities of inputs used in past cropping seasons would be increasingly unreliable and possibly biased as the recall period grows. Furthermore, even if we had information on input quantities, it is not clear how to aggregate these without additional information on application concentrations and relative toxicities. Hence, we used binary variables for consistency. We also did not differentiate between different types of pesticides (e.g., insecticides vs. herbicides vs. fungicides) because pesticide use among farmers in our sample is still rare (Table 1). Both survey instruments are available in the Online Resources 2 and 3.
When both variables are included in the model, both coefficients are statistically insignificant. This is a clear indication for multicollinearity. The results for the other variables in the model are robust to either specification.
In the Richness model (Table 2), the significance levels of the present use of chemical fertilizer and irrigation variables do not depend on whether the variable ‘plot already harvested’ is included.
References
Altermatt F, Ebert D (2016) Reduced flight-to-light behaviour of moth populations exposed to long-term urban light pollution. Biol Let 12(4):20160111
Asfaw S, Di Battista F, Lipper L (2016) Agricultural technology adoption under climate change in the sahel: micro-evidence from Niger. J Afr Econ 25(5):637–669
Banaszak-Cibicka W, Żmihorski M (2012) Wild bees along an urban gradient: winners and losers. J Insect Conserv 16(3):331–343
Basu P, Bhattacharya R, Ianetta P (2011) A decline in pollinator dependent vegetable crop productivity in India indicates pollination limitation and consequent agro-economic crises. Nature Preced. https://doi.org/10.1038/npre.2011.6044.1
Basu P, Parui AK, Chatterjee S, Dutta A, Chakraborty P, Roberts S, Smith B (2016) Scale dependent drivers of wild bee diversity in tropical heterogeneous agricultural landscapes. Ecol Evol 6(19):6983–6992
Baum KA, Wallen KE (2011) Potential bias in pan trapping as a function of floral abundance. J Kansas Entomol Soc 84(2):155–159
Belitz C, Lang S (2008) Simultaneous selection of variables and smoothing parameters in structured additive regression models. Comput Stat Data Anal 53(1):61–81
Blitzer EJ, Dormann CF, Holzschuh A, Klein A-M, Rand TA, Tscharntke T (2012) Spillover of functionally important organisms between managed and natural habitats. Agric Ecosyst Environ 146(1):34–43
Brittain CA, Vighi M, Bommarco R, Settele J, Potts SG (2010) Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl Ecol 11(2):106–115
Catarino R, Bretagnolle V, Perrot T, Vialloux F, Gaba S (2019) Bee pollination outperforms pesticides for oilseed crop production and profitability. Proc R Soc B Biol Sci 286(1912):20191550
Chakrabarti P, Rana S, Sarkar S, Smith B, Basu P (2015) Pesticide-induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two Eastern Indian states. Apidologie 46(1):107–129
Chakraborty P, Chatterjee S, Smith BM, Basu P (2021) Seasonal dynamics of plant pollinator networks in agricultural landscapes: how important is connector species identity in the network? Oecologia 196(3):825–837
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11(4):265–270
Cohen H, Philpott SM, Liere H, Lin BB, Jha S (2021) The relationship between pollinator community and pollination services is mediated by floral abundance in urban landscapes. Urban Ecosyst 24(2):275–290
David TI, Storkey J, Stevens CJ (2019) Understanding how changing soil nitrogen affects plant–pollinator interactions. Arthropod-Plant Interact 13(5):671–684
de Ramos DL, Bustamante MMC, da Silva FDS, Carvalheiro LG (2018) Crop fertilization affects pollination service provision: common bean as a case study. PLoS ONE 13(11):e0204460
Directorate of Census Operations Karnataka (2011) District census handbook, Bangalore (rural) (Census of India 2011 Series-30, Part XII-B)
Elisante F, Ndakidemi PA, Arnold SEJ, Belmain SR, Gurr GM, Darbyshire I, Xie G, Tumbo J, Stevenson PC (2019) Enhancing knowledge among smallholders on pollinators and supporting field margins for sustainable food security. J Rural Stud 70:75–86
Esther Julier H, Roulston TH (2009) Wild bee abundance and pollination service in cultivated pumpkins: farm management, nesting behavior and landscape effects. J Econ Entomol 102(2):563–573
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: patterns and mechanisms. Ann N Y Acad Sci 1223(1):69–81
Gagic V, Bartomeus I, Jonsson T, Taylor A, Winqvist C, Fischer C, Slade EM, Steffan-Dewenter I, Emmerson M, Potts SG, Tscharntke T, Weisser W, Bommarco R (2015) Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc R Soc b: Biol Sci 282(1801):20142620
Goldman RL, Thompson BH, Daily GC (2007) Institutional incentives for managing the landscape: inducing cooperation for the production of ecosystem services. Ecol Econ 64(2):333–343
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229):1255957
Halinski R, Garibaldi LA, dos Santos CF, Acosta AL, Guidi DD, Blochtein B (2020) Forest fragments and natural vegetation patches within crop fields contribute to higher oilseed rape yields in Brazil. Agric Syst 180:102768
Hoffmann EM, Jose M, Nölke N, Möckel T (2017) Construction and use of a simple index of urbanisation in the rural-urban interface of Bangalore, India. Sustainability 9(11):2146
Holzschuh A, Steffan-Dewenter I, Kleijn D, Tscharntke T (2007) Diversity of flower-visiting bees in cereal fields: effects of farming system, landscape composition and regional context. J Appl Ecol 44(1):41–49
Jayne TS, Mason NM, Burke WJ, Ariga J (2018) Review: taking stock of Africa’s second-generation agricultural input subsidy programs. Food Policy 75:1–14
Kleijn D, Winfree R, Bartomeus I, Carvalheiro LG, Henry M, Isaacs R, Klein A-M, Kremen C, M’Gonigle LK, Rader R, Ricketts TH, Williams NM, Lee Adamson N, Ascher JS, Báldi A, Batáry P, Benjamin F, Biesmeijer JC, Blitzer EJ, Potts SG (2015) Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat Commun 6(1):7414
Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc b: Biol Sci 274(1608):303–313
Kremen C, Williams NM, Thorp RW (2002) Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci 99(26):16812–16816
Kremen C, Williams NM, Bugg RL, Fay JP, Thorp RW (2004) The area requirements of an ecosystem service: Crop pollination by native bee communities in California. Ecol Lett 7(11):1109–1119
Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R, Packer L, Potts SG, Roulston T, Steffan-Dewenter I, Vázquez DP, Winfree R, Adams L, Crone EE, Greenleaf SS, Keitt TH, Klein A-M, Regetz J, Ricketts TH (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10(4):299–314
Krishnan S, Kushalappa CG, Shaanker RU, Ghazoul J (2012) Status of pollinators and their efficiency in coffee fruit set in a fragmented landscape mosaic in South India. Basic Appl Ecol 13(3):277–285
Laha S, Chatterjee S, Das A, Smith B, Basu P (2020) Exploring the importance of floral resources and functional trait compatibility for maintaining bee fauna in tropical agricultural landscapes. J Insect Conserv 24(3):431–443
Maertens M, Minten B, Swinnen J (2012) Modern food supply chains and development: evidence from horticulture export sectors in sub-Saharan Africa. Dev Policy Rev 30(4):473–497
Matson PA, Parton WJ, Power AG, Swift MJ (1997) Agricultural intensification and ecosystem properties. Science 277(5325):504–509
Meyer ST, Koch C, Weisser WW (2015) Towards a standardized Rapid Ecosystem Function Assessment (REFA). Trends Ecol Evol 30(7):390–397
Minten B, Koru B, Stifel D (2013) The last mile(s) in modern input distribution: Pricing, profitability, and adoption. Agric Econ 44(6):629–646
Motzke I, Klein A-M, Saleh S, Wanger TC, Tscharntke T (2016) Habitat management on multiple spatial scales can enhance bee pollination and crop yield in tropical homegardens. Agric Ecosyst Environ 223:144–151
Nicholson CC, Koh I, Richardson LL, Beauchemin A, Ricketts TH (2017) Farm and landscape factors interact to affect the supply of pollination services. Agric Ecosyst Environ 250:113–122
Park MG, Blitzer EJ, Gibbs J, Losey JE, Danforth BN (2015) Negative effects of pesticides on wild bee communities can be buffered by landscape context. Proc R Soc b: Biol Sci 282(1809):20150299
Pickett STA, Cadenasso ML, Grove JM, Boone CG, Groffman PM, Irwin E, Kaushal SS, Marshall V, McGrath BP, Nilon CH, Pouyat RV, Szlavecz K, Troy A, Warren P (2011) Urban ecological systems: scientific foundations and a decade of progress. J Environ Manage 92(3):331–362
Priess JA, Mimler M, Klein A-M, Schwarze S, Tscharntke T, Steffan-Dewenter I (2007) linking deforestation scenarios to pollination services and economic returns in coffee agroforestry systems. Ecol Appl 17(2):407–417
Rapoport A (1989) Prisoner’s dilemma. In: Eatwell J, Milgate M, Newman P (eds) Game theory. Palgrave Macmillan, London, pp 199–204
Schwarz B, Vázquez DP, CaraDonna PJ, Knight TM, Benadi G, Dormann CF, Gauzens B, Motivans E, Resasco J, Blüthgen N, Burkle LA, Fang Q, Kaiser-Bunbury CN, Alarcón R, Bain JA, Chacoff NP, Huang S-Q, LeBuhn G, MacLeod M, Fründ J (2020) Temporal scale-dependence of plant–pollinator networks. Oikos 129(9):1289–1302
Shuler RE, Roulston TH, Farris GE (2005) Farming practices influence wild pollinator populations on squash and pumpkin. J Econ Entomol 98(3):790–795
Smith BM, Chakrabarti P, Chatterjee A, Chatterjee S, Dey UK, Dicks LV, Giri B, Laha S, Majhi RK, Basu P (2017) Collating and validating indigenous and local knowledge to apply multiple knowledge systems to an environmental challenge: a case-study of pollinators in India. Biol Conserv 211:20–28
Stallman HR (2011) Ecosystem services in agriculture: determining suitability for provision by collective management. Ecol Econ 71:131–139
Staton T, Walters RJ, Smith J, Girling RD (2019) Evaluating the effects of integrating trees into temperate arable systems on pest control and pollination. Agric Syst 176:102676
Steinhübel L, von Cramon-Taubadel S (2021) Somewhere in between towns, markets and jobs – agricultural intensification in the rural-urban interface. J Dev Stud 57(4):669–694
Tibesigwa B, Siikamäki J, Lokina R, Alvsilver J (2019) Naturally available wild pollination services have economic value for nature dependent smallholder crop farms in Tanzania. Sci Rep 9(1):3434
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418(6898):671–677
Tommasi N, Biella P, Guzzetti L, Lasway JV, Njovu HK, Tapparo A, Agostinetto G, Peters MK, Steffan-Dewenter I, Labra M, Galimberti A (2021) Impact of land use intensification and local features on plants and pollinators in Sub-Saharan smallholder farms. Agric Ecosyst Environ 319:107560
Tscharntke T, Brandl R (2004) Plant-insect interactions in fragmented landscapes. Annu Rev Entomol 49(1):405–430. https://doi.org/10.1146/annurev.ento.49.061802.123339
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity: ecosystem service management. Ecol Lett 8(8):857–874
Tscharntke T, Clough Y, Wanger TC, Jackson L, Motzke I, Perfecto I, Vandermeer J, Whitbread A (2012) Global food security, biodiversity conservation and the future of agricultural intensification. Biol Conserv 151(1):53–59. https://doi.org/10.1016/j.biocon.2012.01.068
Tuell JK, Isaacs R (2010) Community and species-specific responses of wild bees to insect pest control programs applied to a pollinator-dependent crop. J Econ Entomol 103(3):668–675
Turrini T, Knop E (2015) A landscape ecology approach identifies important drivers of urban biodiversity. Glob Change Biol 21(4):1652–1667
Tylianakis JM (2013) The global plight of pollinators. Science 339(6127):1532–1533
Umlauf N, Adler D, Kneib T, Lang S, Zeileis A (2015) Structured additive regression models: an R interface to BayesX. J Stat Softw 63:1–46
Vandercasteelen J, Beyene ST, Minten B, Swinnen J (2018) Cities and agricultural transformation in Africa: Evidence from Ethiopia. World Dev 105:383–399
Viswanathan P, Mammides C, Roy P, Sharma MV (2020) Flower visitors in agricultural farms of Nilgiri Biosphere Reserve: do forests act as pollinator reservoirs? J Apic Res 59(5):978–987
Wenzel A, Grass I, Belavadi VV, Tscharntke T (2020) How urbanization is driving pollinator diversity and pollination: a systematic review. Biol Conserv 241:108321
Wenzel A, Grass I, Nölke N, Pannure A, Tscharntke T (2022) Wild bees benefit from low urbanization levels and suffer from pesticides in a tropical megacity. Agric Ecosyst Environ 336:108019
Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SPM, Szentgyörgyi H, Tscheulin T, Vaissière BE, Woyciechowski M, Biesmeijer JC, Kunin WE, Settele J, Steffan-Dewenter I (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78(4):653–671
Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90(8):2068–2076
Acknowledgements
We thank the editor Prof. Gretchen Walters and two anonymous reviewers for their excellent and very constructive comments. We furthermore thank the entire staff of the Department of Entomology at the University of Agricultural Sciences Bangalore under Prof. Dr. Vasuki Belavadi for their support at all stages of this study and in particular Aarhti Pannure and Yeswanth H. W. for their support in the identification of bees. In addition, our thanks go to our collaborators in the Department of Agricultural Economics under Prof. Chinnappa B. V. Reddy and Prof. K. B. Umesh for setting up the socioeconomic/management surveys as well as the whole field team, in particular Anjali Purushotham, Monish Jose, Radha N., Pavithra S., and Shruthi H. K. We also greatly appreciate the feedback and comments on our study and manuscript provided by Ingo Grass, Teja Tscharntke, and Scott Swinton.
Funding
Open Access funding enabled and organized by Projekt DEAL. The data were collected under the auspices of Research Unit 2432 “Ecological and Social Systems at the Indian Rural–Urban Interface: Functions, Scales and Dynamics of Transition” funded by the German Research Foundation (DFG) (Grant No. 279374797) and the Indian Department of Biotechnology (DBT). This research was also supported by the DFG Research Training Group 1644 "Scaling Problems in Statistics", Grant No. 152112243. In addition, this work was partially supported by the U.S. Department of Agriculture National Institute of Food and Agriculture (USDA-NIFA) and Michigan AgBioResearch (project number MICL02501). The contents are the sole responsibility of the authors and do not necessarily reflect the views of any of the funding agencies mentioned above.
Author information
Authors and Affiliations
Contributions
Conceptualization: LS, AW, SvC-T; Data collection (pan traps): PH, AW; Data collection (farm management): LS; Formal analysis and investigation: LS; Writing—original draft preparation: LS, AW; Writing—review and editing: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The research involved human participants in two household surveys. The interviews were only conducted after the respondents were informed that participation was completely voluntary and could be terminated at any time and after they gave their consent by signing a respective consent sheet (see Online Resources 1 and 2).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steinhübel, L., Wenzel, A., Hulamani, P. et al. Effects of local farm management on wild bees through temporal and spatial spillovers: evidence from Southern India. Landsc Ecol 37, 2635–2649 (2022). https://doi.org/10.1007/s10980-022-01507-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01507-8