Abstract
Context
Coral reef resilience is the product of multiple interacting processes that occur across various interacting scales. This complexity presents challenges for identifying solutions to the ongoing worldwide decline of coral reef ecosystems that are threatened by both local and global human stressors.
Objectives
We highlight how coral reef resilience is studied at spatial, temporal, and functional scales, and explore emerging technologies that are bringing new insights to our understanding of reef resilience. We then provide a framework for integrating insights across scales by using new and existing technological and analytical tools. We also discuss the implications of scale on both the ecological processes that lead to declines of reefs, and how we study those mechanisms.
Methods
To illustrate, we present a case study from Kāneʻohe Bay, Hawaiʻi, USA, linking remotely sensed hyperspectral imagery to within-colony symbiont communities that show differential responses to stress.
Results
In doing so, we transform the scale at which we can study coral resilience from a few individuals to entire ecosystems.
Conclusions
Together, these perspectives guide best practices for designing management solutions that scale from individuals to ecosystems by integrating multiple levels of biological organization from cellular processes to global patterns of coral degradation and resilience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scale is fundamental to our understanding of any system, particularly given that our observations are directly affected by an integration of processes acting at multiple scales (Wiens 1989; Levin 1992) posed that the ‘central problem in ecology’ is that the scale of observations is often different than the scale of the process being studied. Multiple facets of scale need to be considered, including grain and extent (Turner et al. 2001). Grain is the size of individual units of observation, such as a coral polyp or a transect. Extent is the domain of the study, such as a cell or an archipelago. The grain and extent of a study define the limits for which scale-dependent inferences can be drawn, given that information content often correlates with both. Therefore, considering ecosystems as complex hierarchical systems, where biological organization encompasses a wide range of scales, from microbes within individuals to ecosystems connected by dispersal of organisms across thousands of kilometers, allows for a deeper understanding of ecological change and a greater ability to predict and address those changes (Allen and Starr 1982; Wiens 1989; Peterson et al. 1998).
Coral reefs are complex hierarchical systems that host a wide diversity of marine life and provide vital ecosystem services (Moberg and Folke 1999; Hoegh-Guldberg et al. 2019), but are threatened worldwide by multiple local and global stressors (Jackson et al. 2001; Pandolfi et al. 2003; Hughes et al. 2017). Critical to understanding the impact of these stressors is our ability to measure change, which can manifest on different spatial, temporal, and functional scales. Examples include shifts in the relative abundance of species and their size classes, to wholesale differences in the functioning of the ecosystem. Much of what has been observed over the past 70 years of coral reef research has focused on the relative spatial cover of corals (Hughes et al. 2010), given their foundational role as habitat generators and ecosystem engineers. However, ecological change is occurring at multiple scales of biological organization, and modern technologies are now rapidly improving our ability to assess changes on reefs across scales that range from cellular to global (Calders et al. 2020).
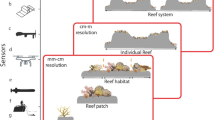
One property of complex systems that determines ecosystem change is resilience, which can be viewed as ecological resilience—the capacity of an ecosystem to withstand disturbance without changing its overall identity in terms of structure and function (Holling 1973; Gunderson 2000; Nyström et al. 2008)—or from a focus on stability, termed engineering resilience, which can be measured as the speed of recovery, or return to equilibrium, following a disturbance (Holling and Meffe 1996). Resilience on coral reefs can scale from the physiology of individual organisms, to the persistence of an entire reef, to the broader linked social-ecological system (Jackson 1991; Hatcher 1997; Nyström et al. 2008; Roche et al. 2018) (Fig. 1). For example, studying the regulation of proteins, enzymes, and individual genes across the coral holobiont (i.e., the coral animal, Symbiodiniaceae, and the microbiome) is needed to understand resistance to stressors (Bourne et al. 2016; Bay et al. 2017). Predicting the potential for selection can be further understood at the grain size of a population. For example, as disturbances cause differential mortality of susceptible coral colonies, resistant genotypes will persist, increasing the frequency of beneficial genes or alleles in the population. Further implications of resilience are evidenced at the scale of ecological communities, including the well-referenced example of coral to macroalgal phase shifts of reef benthic communities (Hughes 1994). Beyond the scale of a community, reefs are connected through larval dispersal by processes which interact with climate and biophysical gradients to establish the connectivity of multiple reefs within and among archipelagos. Thus, each of these scales can be explored at multiple grain sizes and extents using a variety of tools and observations designed to understand the underlying processes and interactions driving local and global change.
Multiple spatial and biological scales used to study reef processes and dynamics that can vary in extent and grain exemplified for Montipora capitata in Kāneʻohe Bay, Hawaiʻi, USA [Image credits: (Region—Reef) ESRI basemap imagery, (Pixel) Joshua Levy, (Ecosystem, Population-Polyp) Raphael Ritson-Williams, (Community) Ingrid Knapp, (Cell)–Shayle Matsuda]
Here we explore how coral reef resilience could be better understood by bridging investigations across multiple levels of biological organization and scales (Fig. 1). We review how data are collected at each level, highlight recent technological advances, and discuss implications for understanding cross-scale phenomena. Additionally, we present a framework for drawing inference across multiple measures and multiple scales, ranging from polyps to pixels, using a case study in Kāneʻohe Bay, Hawaiʻi, USA. This perspective provides a framework for bridging between molecular studies and remote sensing to tell a more complete story that better describes coral reef resilience through the lens of cross-scale resilience theory.
Tools and advances across scales of biological organization
Cell
‘Omics’ tools (e.g., genomics, epigenomics, transcriptomics, proteomics and metabolomics) are at the forefront of the study of the intracellular aspects of coral. These tools have greatly increased our understanding of fine-scale population connectivity and genetic structure, adaptation and acclimatization to environmental change, symbiont community dynamics, and sublethal responses to environmental stressors, disease, and recovery (Vega Thurber et al. 2009; Barshis et al. 2013; Dixon et al. 2015; Seneca and Palumbi 2015; Voolstra et al. 2015; Kenkel and Matz 2016; Putnam et al. 2016; Forsman et al. 2020; Roach et al. 2021). For example, such approaches have been used to determine the genomic basis of coral resilience to climate change (i.e., what genes underlie thermal tolerance) and to what degree thermotolerance is driven by genetic adaptation versus physiological plasticity (Barshis et al. 2013; Palumbi et al. 2014). These advances enable the selection of thermotolerant genotypes for coral restoration and other human interventions to increase coral persistence under climate change, such as assisted gene flow or translocation (National Academies of Sciences, Engineering, and Medicine 2019a). ‘Omics’ tools have the advantage that only a small tissue sample or even single cells collected in the field or lab can generate an immense amount of data (e.g., millions of sequence-reads per individual). This convenience is balanced by the need for specialized expertise and equipment, high costs of lab preparation and sequencing [e.g., Restriction-site Associated DNA Sequencing (RAD-Seq) and RNAseq] along with the development of bioinformatic pipelines, and computational constraints of big data generation.
Multiple advances in cellular studies of corals are paving a path towards greater applications to conservation solutions. First, sequence data and metadata are typically deposited in freely and publicly available repositories [e.g., NCBI Sequence Read Archive (Leinonen et al. 2010)], offering potential avenues for broader research endeavors spanning across coral species and multiple stressors. Technological advances also continue to reduce costs of both sequencing and computation of sequence data. Together, these advances will lead to greater availability and application of these tools as the field continues to progress. Nonetheless, small sample sizes at this level of biological organization likely under-represent the functional and genetic diversity of corals and conditions, and thus a scale-gap occurs in making broad inferences from such localized data.
Polyp
Single polyps originating from planula larvae form the ‘individual’ level of biological organization for corals, and many polyps together form a coral colony. Studies at the larval and polyp scale are key to understanding fundamental biological processes in corals, such as settlement and metamorphosis, establishment and maintenance of symbiosis, and formation of calcium carbonate skeletons, which in turn govern resilience at higher levels of organization. Polyp biology has largely been informed by studying coral reproduction (Harrison 2011), where researchers typically collect coral gametes in the field and conduct laboratory experiments to understand patterns of fertilization, dispersal, settlement, and post-settlement ecology (Ritson-Williams et al. 2009). Research on coral larvae spans from behavior (Dixson et al. 2014), physiology (Gleason and Hofmann 2011), and ecology (Ritson-Williams et al. 2016) to genomics and transcriptomics (Polato et al. 2013; Kirk et al. 2018; Fuller et al. 2020), using many of the same techniques applied to the cellular processes described earlier.
Studying coral polyps and larvae has generally been limited to laboratory experiments because of their small size and the low probability of observing settlement and early post-settlement life stages in situ (but see Carlon and Olson 1993). However, hundreds to thousands of larvae can be grown and settled in the laboratory (Ritson-Williams et al. 2016), and recent technological advances are increasing the success and accessibility of spawning corals in ex situ closed mesocosms (Craggs et al. 2017), accelerating research at this scale. In situ sampling of established recruits over time for population genetic analyses, however, could enable integration over multiple time scales, allowing us to track changes in genotypic diversity if a habitat is surveyed repeatedly before and after a disturbance event. Further, there is evidence that symbionts (and by proxy, physiologic resistance to stress) can vary across microhabitats found within a single coral colony (Rowan et al. 1997), and there have been recent advances in single cell ‘omic techniques that allow for measuring variation across polyps within a holobiont, so we expect that our understanding of polyp level biology as it relates to aspects of coral resilience will greatly increase in the near future.
Holobiont
The coral colony is composed of many individual polyps that, together with the microbiome and symbiotic microalgae in the family Symbiodiniaceae (LaJeunesse et al. 2018), form the coral holobiont. Individual holobionts have variable responses to stress (Barshis et al. 2013; Drury et al. 2017; Ritson-Williams and Gates 2020). This variability in stress response was classically studied using ecology and physiology (Edmunds and Gates 2008) but has recently progressed using transcriptomics (Kenkel and Matz 2016; Kirk et al. 2018), genomics (Bay and Palumbi 2014; Howells et al. 2016; Fuller et al. 2020), and microbiology (Bourne et al. 2016). Genetic techniques have greatly increased our knowledge of the diversity of coral-associated symbionts and microbes (Rowan and Powers 1992; Vega Thurber et al. 2009; Hernandez-Agreda et al. 2017; LaJeunesse et al. 2018), and many studies have demonstrated how coral holobiont performance and resilience are linked to the composition and diversity of Symbiodiniaceae (Iglesias-Prieto et al. 1992; Glynn et al. 2001; Berkelmans and Van Oppen 2006; LaJeunesse et al. 2010; Cunning et al. 2016; Hume et al. 2019), other microeukaryotes (Kwong et al. 2019), bacteria (Ziegler et al. 2017; Boilard et al. 2020), and viruses (Vega Thurber et al. 2017).
The clonal properties of corals provide an advantage for research such that genetically identical fragments of the same colony can be used as replicates in experimental manipulations. Importantly, while the identity of the coral animal may remain fixed across these replicate fragments, microbial partners may vary (Rowan et al. 1997), and can even be directly manipulated to study and/or generate specific holobiont combinations and phenotypes, including stress tolerance (Rosado et al. 2019; Cunning and Baker 2020). However, microbial manipulations can be limited in both grain and extent, and therefore may not translate well to broader scales. Advances in studying coral microbiomes, transcriptomics, and genomics will allow for increasing the extent of studies on the variability of coral holobionts, demonstrating the research potential at this organizational scale. Mathematical modeling approaches applied to coral-symbiont interactions (e.g., dynamic energy budget theory; (Muller et al. 2009; Cunning et al. 2017), may also help to mechanistically link environmental impacts on the coral holobiont to higher levels of biological organization (e.g., Martin et al. 2013).
Population
Population-level analyses of corals have improved our understanding of coral resilience through identification of cryptic species (Rose et al. 2018; Forsman et al. 2020; Burgess et al. 2021), inferred mechanisms of adaptive potential (Knowlton and Leray 2015; Bay et al. 2017), characterization of relationships among populations and dispersal patterns (Toonen et al. 2011; Drury et al. 2018; Matz et al. 2018), and attribution of key processes driving population dynamics (Madin and Connolly 2006; Roth et al. 2010; Hughes et al. 2019; Dietzel et al. 2021). Population-level molecular analyses and coral genotyping have informed predictions of future impacts of environmental change (Selkoe et al. 2016; Bay et al. 2017; Underwood et al. 2018; Fuller et al. 2020), and resistance to disease (Vollmer and Kline 2008). Population dynamic studies of corals have revealed the importance of variation in recruitment (Hughes and Tanner 2000; Edmunds et al. 2010) and overall size structure (Bak and Meesters 1999; Dietzel et al. 2020, 2021) in determining resistance and recovery from disturbance.
Despite early calls for coral demography to be the center of coral population studies (Connell 1973; Hughes 1984), the need to move beyond measures of percent cover for corals and include coral demography are still being echoed today (Edmunds and Riegl 2020). Further, measures of vital rates (e.g., growth, survival, fecundity), and their incorporation in modeling the future trajectory of corals is understudied. One challenge to demographic studies is the complex life history of corals that involve a variety of reproductive strategies and complex growth, but also fission, fusion, and shrinkage (Edmunds and Riegl 2020). Advances in photogrammetry and 3-dimensional modeling of coral reef systems are providing new pathways for studying coral population dynamics (Burns et al. 2015) (Fig. 2), and in particular coral size frequency (Burns et al. 2016; Hernández-Landa et al. 2020). This emerging method can enable us to better estimate the complex 3-dimensional structures made by corals without the need for in situ observations, expanding how we estimate coral resilience in a changing world.
3D modeling of a coral reef system across multiple spatial scales. A Close-up of an area within the Waiʻōpae coastline on Hawai‘i island showing individual coral colonies with inset map showing spatial coverage of the survey area reconstructed in 3D using aerial images. B Oblique view of the 3D model of the same location (m-resolution), with IVAN multirotor aerial Unmanned Aircraft Vehicle (UAV) platform shown in inset photo. C Oblique view of a 3D model of coral colonies (mm-resolution) and D a 3D model of an individual colony (mm-resolution) reconstructed from this same location, both generated with underwater Structure from Motion (SfM) photogrammetry techniques (Image credit: Burns and Perroy, UH Hilo)
Community
Community-level studies of corals reefs have increased our understanding of how biotic interactions, diversity, and the role of corals as habitat engineers relate to reef resilience. Biotic interactions can play a major role in driving coral community composition through competition (e.g., among corals and algae), predation, and ecological feedbacks such as herbivory effects. The role of interactions between corals, algae, and herbivores in shaping reef resilience in particular has received considerable attention (Nyström and Folke 2001; Bellwood et al. 2004; Mumby et al. 2007; Hughes et al. 2010; Steneck et al. 2018), as herbivory has been identified as a key process determining whether reefs might rapidly transition from coral dominated to algae dominated reefs (Hughes et al. 2007; Mumby et al. 2007; Burkepile and Hay 2008; Steneck et al. 2019). Community-level studies also include the implications of diversity (Warwick and Clarke 1990; Paulay 1997), and coral community structure (Loya et al. 2001; Hughes et al. 2018) on resilience. Studies on coral assemblage effects on the broader reef community have also been critical for understanding reef resilience, such as the ecological effects of coral declines on fish assemblages (Pratchett et al. 2008; Fukunaga et al. 2020), and how reduced structural complexity leads to broad scale community change (Alvarez-Filip et al. 2009).
Compared to other levels of biological organization on coral reefs, studying benthic communities can be done relatively inexpensively with simple equipment and methods. Basic metrics such as percent cover of coral can be obtained relatively rapidly and across large geographic scales by researchers and community (formally termed “citizen”) scientists alike (Aronson et al. 1994; Hodgson 1999; Stuart-Smith et al. 2017). However, the simplicity of the tools needed belies the importance of an in-depth knowledge of the natural history of the focal community, often integrating an understanding of each of the other levels of biological organization discussed here. Similarly, given the lack of standardized methodologies through the relatively long history of monitoring coral communities, comparing data sets across spatial and temporal scales can be challenging (Jackson et al. 2014). Our understanding of coral communities is advancing through new approaches including in situ and low altitude aerial photogrammetry (Burns et al. 2015; Levy et al. 2018), and aerial imagery (Asner et al. 2020) that provide repeatable, unbiased, high-resolution data at broader spatial extents than previously possible. Data acquisition from these emerging imaging capabilities are also being made possible from innovation in automated image analysis tools, such as CoralNet (Beijbom et al. 2015; Williams et al. 2019).
Ecosystem
Studies of corals at the ecosystem level involve examining spatial variation and temporal trends across extents and/or grain sizes at or larger than individual coral communities (Fig. 1). These broad scale data can assess resiliency of a system to pulse events such a marine heatwaves and cyclones (Stuart-Smith et al. 2018), as well as press disturbances like habitat degradation from land-based pollution (Palandro et al. 2008). Ecosystem-scale data are particularly important for understanding context, and for extrapolating finer resolution biotic responses discovered in ‘omic, holobiont, population, and community studies for understanding resiliency at management-relevant spatial scales. Remotely sensed data sources (e.g., multispectral and hyperspectral imagery, LiDAR, SONAR) have emerged as a key source of data for ecosystem-level observations that include the ability to measure depth (Salameh et al. 2019), habitat complexity (Lepczyk et al. 2021), environmental drivers like sea surface temperature and turbidity (El Mahrad et al. 2020), and more recently live coral cover at broad extents (Asner et al. 2020). While live coral cover mapping is currently limited to aircraft-based imaging spectrometers, forthcoming spaceborne spectrometers from NASA, the European Space Agency, and the private sector will make this capability globally accessible. Until then, space-based observation of broad benthic and geomorphic reef compositions from multispectral satellites will continue (Hedley et al. 2016; Li et al. 2020).
Resolution and extent of remotely sensed data are inversely correlated given the logistical costs of data collection, which limits the scope of what can be investigated for any individual mapping effort. Further, gaps in the satellite record for different types of data can be troublesome especially for analyses over time. For example, sea temperature datasets only date back to the 1980s [e.g., the Coral Reef Temperature Anomaly Database (CoRTAD) (Saha et al. 2018)], which presents uncertainty in retrospective analyses of heatwaves. Despite these challenges, uptake of remotely sensed data in coral research is rapidly growing as both spectral and radiometric resolution improves from both satellite and aerial sensors. Further, use of high quality, reliable ecosystem-level information for coral management is increasing as data becomes more available through efforts such as the Allen Coral Atlas (https://allencoralatlas.org) that are making information on coral reef habitat composition, bleaching, and other products available for the entire globe (Li et al. 2020, 2021).
Human and physical drivers of reefs across scales
Coral reefs are affected by multiple physical and anthropogenic drivers, such as pollution, overfishing, and climate change (Jackson et al. 2001; Fabricius 2005; Hughes et al. 2017). These drivers often do not occur in isolation and can therefore result in additive, synergistic, or antagonistic responses across multiple scales (Côté et al. 2016). For example, variable bleaching responses to increased temperature can occur within a single colony (Rowan et al. 1997), among colonies of the same species within a population (Jones 2008; Williams et al. 2010; Ritson-Williams and Gates 2020), or across different species within a community (van Woesik et al. 2011). At the ecosystem level, biophysical influences on benthic cover can vary at the scale of islands (Williams et al. 2015) and within small islands; for example, wave forcing and geomorphology can predict benthic regimes at the scale of 100 s of meters (Gove et al. 2015; Aston et al. 2019).
When examining how drivers interact and influence biological outcomes on coral reefs, the implication of scale must be considered (Turner et al. 2001). Yet, biological response and driver data are not necessarily measured at the same scale. For example, there is often a disconnect between fine scale (spatial or temporal) temperature variation, measured with in situ loggers (< 1 m/every 20 min), verses global satellite derived temperature (4 km/twice weekly) and global climate model outputs (100 km2/monthly) (Safaie et al. 2018). Human influences on coral reefs are also manifested at a variety of spatial and temporal scales, and these multi-stressor relationships can be masked if studied at differing grain or extent sizes than those at which the processes occur. For example, Jouffray et al. (2019) uncovered important biophysical and human influences on coral reefs in Hawai‘i, and did not detect any differences in their results after repeating analyses at multiple grain sizes. In particular, the study did not uncover effects of land-based pollution despite well understood connections between pollution and reef condition (Fabricius 2005), which was hypothesized to be a result of the grain size of the pollution data not matching the highly localized scale at which pollution affects reefs. Development and use of unmanned systems (Obura et al. 2019), sensor networks (Trevathan et al. 2012), and low-cost cameras (Greene et al. 2020) are attempting to bridge the gap between observable biological responses and environmental driver data at scales relevant for improving our understanding of these complex systems.
One limitation to linking drivers and observations at appropriate scales is a lack of evidence for the scale at which the processes are occurring. For example, there is some evidence that scaling patterns are common for coral communities in relation to biophysical gradients that may allow for a common scaling law for coral reef benthic communities on island seascapes (Gove et al. 2015; Aston et al. 2019). However, the evidence for scaling in benthic reef communities comes from remote Pacific islands with relatively low human impacts, and relationships may break down when human influences disrupt natural processes. For example, Williams et al. (2015) found that across the Pacific homogenized reefs with low diversity and a high abundance of ‘weedy’ species common on populated islands were not coupled with background environmental regimes compared to strong biophysical coupling observed on unpopulated islands. Indeed, physical and human influences on coral reef systems can be evident in different contexts (Cinner et al. 2018; Jouffray et al. 2019); therefore, interactions among human and physical drivers need to be better understood in order to define boundaries for conservation and restoration. Difficulty uncovering appropriate scales for inquiry is further compounded by the potential for cross-scale phenomena, where processes could be occurring across and within scales (Peters et al. 2007). Cross-scale redundancy has been shown to be an indicator of resilience, where better recovery after disturbance was observed for reefs with herbivory operating across multiple scales (Nash et al. 2016). Multiscale problems also may require potentially arbitrary delineations of discrete scales (Levin 1992), potentially masking the underlying processes. Thus, future research needs to explicitly incorporate scale in order to understand how human and biophysical drivers interact to influence reef status and trends.
Linking observations across scales
Given the range of tools used to gain insight into reef resilience, and the scales of stressors on corals, it is critical to link our understanding of processes that allow for coral resilience across scales so that we may better predict how corals will respond to changing environmental conditions. For example, much of what we know about bleaching resistance of corals comes from molecular studies and manipulative tank experiments, both of which are logistically limited in size and breadth. But understanding how bleaching resistance is manifested in populations, communities, and ecosystems is fundamental to both informing future predictions and designing effective management for corals.
For example, symbiont communities of corals are an important factor relating to many biological and ecological outcomes on coral reefs (Baker et al. 2008). Different genera of symbionts influence the thermal tolerance of the host coral, so the composition and relative abundance of symbiont taxa is particularly important for the long-term persistence of coral reef ecosystems (Berkelmans and Van Oppen 2006; Logan et al. 2021). Various molecular biology techniques can resolve these patterns, but time, cost, and accessibility limit the availability of these data to relatively few well-studied ecosystems. Here, we provide an example of how the differential responses to heat stress by individual corals with different symbionts can be predicted via remotely-sensed hyperspectral imagery. We show that symbiont community can be linked to hyperspectral imagery through color morphs in Montipora capitata, effectively transforming the scale at which we examine symbiosis ecology from the within polyp-scale to the whole reef-scale (Fig. 3).
Previous molecular genetic studies of M. capitata holobionts in Kāneʻohe Bay, Oʻahu, Hawai‘i, revealed two color morphs, brown and orange, which were associated with Cladocopium and Durusdinium symbionts, respectively. The color morph and symbiont were correlated with differential responses to a major bleaching event (Cunning et al. 2016; Innis et al. 2018), with orange, Durusdinium-dominated colonies showing much greater bleaching resistance. When combining this observation with high-fidelity imaging spectroscopy collected by aircraft through the Global Airborne Observatory (Asner et al. 2012), we were able to successfully distinguish between individual corals of the two color morphs at a scale of an entire patch reef (Fig. 3). By connecting the biology of individual corals studied in situ using ‘Omics techniques with remotely sensed data at broad scales, we link coral physiology at the scale of individuals and the scale of seascapes, showing that differential responses to stress at a physiological level can be predicted at a seascape scale. Thus, we provide an example of how cross-scale study could revolutionize our understanding and management of coral reefs given that these inferences can be scaled up to entire islands or archipelagos to provide actionable science on how the potential resilience of coral populations to climate-driven disturbances can vary spatially. For example, using remotely sensed data to identify thermotolerant individuals can be used for locating heat tolerant genotypes for use in restoration (Drury et al. 2022). Further, transcriptomic and physiological measurements are being used around the world to rapidly identify thermally resilient coral colonies in situ (Cunning et al. 2021; Naugle et al. 2021; Savary et al. 2021). Combining these data with 3D photogrammetry or remotely sensed data over multiple years encompassing thermal disturbance events, when fine-scale physiological or ‘omics sampling may not feasible, could help in understanding the long-term fate of these thermally “tough” colonies over ecologically and management relevant spatial and temporal scales (Little et al. 2021).
Spectral analysis of Montipora capitata color morphs in Kāneʻohe Bay. A Sampling points at Reef 44 where benthic reflectance was retrieved from brown (n = 25) and orange (n = 25) color morphs. B Illustrative region with neighboring orange and brown color morphs which were C defined as each color based on high resolution Global Airborne Observatory imagery. D The brightness normalized spectra of brown and orange color morphs show different reflectance at various wavelengths which can be used in discriminant analysis, E correctly classifying 64% of samples (Image credit: G. Asner and J. Heckler, Global Airborne Observatory). For details on methods see Supplemental Material
In addition to emerging technologies transforming the way we study coral resilience, advances in how data from multiple scales can be brought together for new inferences is possible through the increasing use of hierarchical modeling in ecology and conservation (Bolker et al. 2009; MacNeil and Connolly 2015). Hierarchical models allow for explicitly incorporating hypotheses and data at multiple scales in an integrated form, due to the ability to formulate flexible nested model structures. Thus, finer scale patterns and processes can be understood in the context of broader scale phenomena. For example, data collected at a fine scale on the response of a coral to disturbance may be combined with predictors that correspond to multiple hierarchical scales and can be incorporated at the scale at which the relationship to the coral’s response is hypothesized. MacNeil et al. (2009) illustrate how this statistical framework can be used to uncover how fish abundance and habitat diversity vary in relation to site-, reef-, and atoll-scale processes using hierarchical models that considered data at each scale. Since this publication, and a subsequent call for this to become a major research avenue for reef research (Hixon 2011), these methods have yet to be adopted by coral reef scientists globally. Therefore, the use of hierarchical models in linking fine- and broad-scale ecosystem processes holds great promise for furthering our understanding of reef resilience (MacNeil et al. 2015; Cinner et al. 2016; Donovan et al. 2020).
One path forward for coral reef resilience studies is to use emerging technologies and quantitative frameworks to translate how the physiological and behavioral responses of individuals scale to the seascape, and ultimately to the benefits and services reefs provide to people. We demonstrate that this is possible via data collected across scales in Kāneʻohe Bay, Oʻahu, Hawaiʻi, USA, but that is just the beginning.
Applications for effectively managing and conserving reefs
Given that the resilience of coral reefs is manifested across multiple scales of biological organization, and that feedbacks can exist among those scales, it is critical to link ideas and insights from the cellular-to-polyps-to-ecosystem-levels in order to effectively manage and conserve coral reefs in the face of local and global change.
Matches and mismatches exist between the scale at which we measure coral reef resilience (Fig. 4A), and the scales at which resilience is manifested, thereby posing serious challenges to coral reef conservation and management. These challenges exist because the varying spatial and temporal scales at which human impacts affect coral reefs (Fig. 4B) are often misaligned with the scales of management policy and action (Fig. 4C) (Cumming et al. 2006; Bellwood et al. 2019). To address the challenges from this scale-dependent mismatch, resilience-based management has emerged (Mcleod et al. 2019), including broad strategies that are adaptive and flexible and employ both interventionist and restoration-based tactics. Importantly, resilience-based management acknowledges upfront that systems are scale-dependent, and an understanding of scale needs to be incorporated into solutions (Mcleod et al. 2019).
Despite the emergence of resilience-based management strategies, global climate change only exacerbates the scale-dependent mismatches between coral reef resilience management and human impacts. Coral reef management is most often local in scale and limited to jurisdictions in which reefs occur; thus, addressing global scale stressors is often beyond the purview of those that are most affected. One path forward includes regional international partnerships between countries that share coral reef habitats, providing a platform for federal governments and conservation agencies to work together to meet coral reef resilience goals. The MAR Fund (https://marfund.org/) is an example of such a partnership that provides support for the countries bordering the Mesoamerican Barrier Reef in the Caribbean: Mexico, Belize, Guatemala and Honduras. Potential improvements in reef futures are possible by building off of these local and regional initiatives to bolster reef resilience alongside concerted efforts to curb global climate change. There is growing evidence that mitigating local stressors such as fishing and pollution can increase the potential for corals to withstand climate effects (Gilmour et al. 2013; Claar et al. 2020; Donovan et al. 2020, 2021). For example, nutrient pollution and overfishing of herbivores can both lead to increases in macroalgae, which causes greater mortality of corals following heatwaves (Donovan et al. 2021).
Coral restoration and human interventions provide another path forward for reef futures (Bay et al. 2019; National Academies of Sciences, Engineering, and Medicine 2019b). There have been advancements in managed selection (Van Oppen et al. 2015), such as propagating or translocating heat tolerant corals (Barott et al. 2021; Drury and Lirman 2021), assisted symbiont shuffling (e.g., promoting symbiosis with heat tolerant symbionts) (Buerger et al. 2020), genetic manipulation (Cleves et al. 2020), and altering coral microbiomes (e.g., treating corals with probiotics) (Rosado et al. 2019). Applications of these methods are being adopted by coral restoration projects, which often focus on rearing fragments of corals in nurseries and out-planting to select reef sites to encourage population-level recovery. However, the ability of these methods to scale to ecosystem level restoration is unclear (Boström-Einarsson et al. 2020; Hein et al. 2020) and more work is needed to integrate out-planting with existing ecological and physical bounds of the system (Ladd et al. 2019). Examples using synoptic-scale observations to enhance coral restoration projects includes linking coral outplanting successes and failures to remotely sensed drivers on a global scale (Foo and Asner 2021), and using live coral and algal mapping for restoration site selection (Schill et al. 2021).
A path forward
Due to the wide variety of local and regional stressors to coral reefs, and the mismatch that often occurs between these impacts and management efforts, we propose that future coral reef science activities consider the multi-scale dynamics of coral reef systems, and embrace emerging technologies and methods to address reef science for conservation and management. One way to approach this challenge may be to (i) aggregate mechanistic biological principles from finer scales (e.g., intra-holobiont organismic or chemical interactions) into (ii) species-level performance principles and models at coral population levels, and then to (iii) integrate those populations at community levels through competitive models for space, and then (iv) use the emerging high-resolution remote sensing to constrain the patterns of changing communities in space and time. While most of these individual undertakings are currently limited to research and development that precludes full utilization of an interlinked approach, setting an agenda now for achieving these linkages is fundamental to the longer-term goals and milestones required to scale up coral biology to the ecosystem level at which management interventions are sought.
There have been substantial advances in how we study coral resilience and what has been learned at both the finest grains (i.e., molecular and physiology) and at the broadest (i.e., remote sensing). Thus, the future of coral reef science lies at the intersection. Here, we have provided one such example of how within-individual bleaching resistance was transformed to a seascape scale (Fig. 3). Integration across scales of coral biology and ecology is the future of reef science, and in order for this to be possible, ideas and data must flow across and within disciplines making new and bigger discoveries possible.
Data availability
not applicable.
Code availability
not applicable.
References
Allen T, Starr T (1982) Hierarchy: perspectives for ecological complexity. University of Chicago Press, Chicago, p 326
Alvarez-Filip L, Dulvy NK, Gill JA et al (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc R Soc Lond B Biol Sci 276:3019–3025
Aronson RB, Edmunds PJ, Precht WF et al (1994) Large-scale, long-term monitoring of Caribbean coral reefs: simple, quick, inexpensive techniques. Atoll Res Bull 421(421):1–19
Asner GP, Knapp DE, Boardman J et al (2012) Carnegie Airborne Observatory-2: Increasing science data dimensionality via high-fidelity multi-sensor fusion. Remote Sens Environ 124:454–465
Asner GP, Vaughn NR, Heckler J et al (2020) Large-scale mapping of live corals to guide reef conservation. Proc Natl Acad Sci 117:33711–33718
Aston EA, Williams GJ, Green JAM et al (2019) Scale-dependent spatial patterns in benthic communities around a tropical island seascape. Ecography (Cop) 42:578–590
Bak RPM, Meesters EH (1999) Population structure as a response of coral communities to global change. Am Zool 39:56–65
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Barott KL, Huffmyer AS, Davidson JM et al (2021) Coral bleaching response is unaltered following acclimatization to reefs with distinct environmental conditions. Proc Natl Acad Sci USA 118:e2025435118
Barshis DJ, Ladner JT, Oliver TA et al (2013) Genomic basis for coral resilience to climate change. Proc Natl Acad Sci 110:1387–1392
Bay LK, Rocker M, Boström-Einarsson L et al (2019) Reef Restoration and Adaptation Program: Intervention Technical Summary. A report provided to the Australian Government by the Reef Restoration and Adaptation Program
Bay RA, Palumbi SR (2014) Multilocus adaptation associated with heat resistance in reef-building corals. Curr Biol 24:2952–2956
Bay RA, Rose NH, Logan CA, Palumbi SR (2017) Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Sci Adv 3:e1701413
Beijbom O, Edmunds PJ, Roelfsema C et al (2015) Towards automated annotation of benthic survey images: variability of human experts and operational modes of automation. PLoS ONE 10:e0130312
Bellwood D, Hughes T, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bellwood DR, Pratchett MS, Morrison TH et al (2019) Coral reef conservation in the Anthropocene: confronting spatial mismatches and prioritizing functions. Biol Conserv 236:604–615
Berkelmans R, Van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’for coral reefs in an era of climate change. Proc R Soc B Biol Sci 273:2305–2312
Boilard A, Dubé CE, Gruet C et al (2020) Defining coral bleaching as a microbial dysbiosis within the coral holobiont. Microorganisms 8:1682
Bolker BM, Brooks ME, Clark CJ et al (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Boström-Einarsson L, Babcock RC, Bayraktarov E et al (2020) Coral restoration–A systematic review of current methods, successes, failures and future directions. PLoS ONE 15:e0226631
Bourne DG, Morrow KM, Webster NS (2016) Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu Rev Microbiol 70:317–340
Buerger P, Alvarez-Roa C, Coppin CW et al (2020) Heat-evolved microalgal symbionts increase coral bleaching tolerance. Sci Adv 6:eaba2498
Burgess SC, Johnston EC, Wyatt ASJ et al (2021) Response diversity in corals: hidden differences in bleaching mortality among cryptic Pocillopora species. Ecology 102(6):e03324
Burkepile DE, Hay ME (2008) Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci 105:16201–16206
Burns JHR, Delparte D, Gates RD, Takabayashi M (2015) Integrating structure-from-motion photogrammetry with geospatial software as a novel technique for quantifying 3D ecological characteristics of coral reefs. PeerJ 3:e1077
Burns JHR, Delparte D, Kapono L et al (2016) Assessing the impact of acute disturbances on the structure and composition of a coral community using innovative 3D reconstruction techniques. Methods Oceanogr 15:49–59
Calders K, Phinn S, Ferrari R et al (2020) 3D imaging insights into forests and coral reefs. Trends Ecol Evol 35:6–9
Carlon DB, Olson RR (1993) Larval dispersal distance as an explanation for adult spatial pattern in two Caribbean reef corals. J Exp Mar Bio Ecol 173:247–263
Cinner JE, Huchery C, MacNeil MA et al (2016) Bright spots among the world’s coral reefs. Nature 535:416–419
Cinner JE, Maire E, Huchery C et al (2018) Gravity of human impacts mediates coral reef conservation gains. Proc Natl Acad Sci USA 115:E6116–E6125
Claar DC, Starko S, Tietjen KL et al (2020) Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nat Commun 11:1–10
Cleves PA, Tinoco AI, Bradford J et al (2020) Reduced thermal tolerance in a coral carrying CRISPR-induced mutations in the gene for a heat-shock transcription factor. Proc Natl Acad Sci USA 117:28899–28905
Connell JH (1973) Population ecology of reef-building corals. Biol Geol Coral Reefs 2:205–245
Côté IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc R Soc B Biol Sci 283:20152592
Craggs J, Guest JR, Davis M et al (2017) Inducing broadcast coral spawning ex situ: closed system mesocosm design and husbandry protocol. Ecol Evol 7:11066–11078
Cumming GS, Cumming DHM, Redman CL (2006) Scale mismatches in social-ecological systems: causes, consequences, and solutions. Ecol Soc 11:1–20
Cunning R, Baker AC (2020) Thermotolerant coral symbionts modulate heat stress-responsive genes in their hosts. Mol Ecol 29:2940–2950
Cunning R, Muller EB, Gates RD, Nisbet RM (2017) A dynamic bioenergetic model for coral-Symbiodinium symbioses and coral bleaching as an alternate stable state. J Theor Biol 431:49–62
Cunning R, Parker KE, Johnson-Sapp K et al (2021) Census of heat tolerance among Florida’s threatened staghorn corals finds resilient individuals throughout existing nursery populations. Proc R Soc B 288:20211613
Cunning R, Ritson-Williams R, Gates RD (2016) Patterns of bleaching and recovery of Montipora capitata in Kāne ‘ohe Bay, Hawai ‘i, USA. Mar Ecol Prog Ser 551:131–139
Dietzel A, Bode M, Connolly SR, Hughes TP (2020) Long-term shifts in the colony size structure of coral populations along the Great Barrier Reef. Proc R Soc B 287:20201432
Dietzel A, Bode M, Connolly SR, Hughes TP (2021) The population sizes and global extinction risk of reef-building coral species at biogeographic scales. Nat Ecol Evol 5:663–669
Dixon GB, Davies SW, Aglyamova GV et al (2015) Genomic determinants of coral heat tolerance across latitudes. Science 348:1460–1462
Dixson DL, Abrego D, Hay ME (2014) Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345:892–897
Donovan MK, Adam TC, Shantz AA et al (2020) Nitrogen pollution interacts with heat stress to increase coral bleaching across the seascape. Proc Natl Acad Sci USA 117:5351–5357
Donovan MK, Burkepile DE, Kratochwill C et al (2021) Local conditions magnify coral loss after marine heatwaves. Science 372:977–980
Drury C, Lirman D (2021) Genotype by environment interactions in coral bleaching. Proc R Soc B 288:20210177
Drury C, Manzello D, Lirman D (2017) Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLoS ONE 12:e0174000
Drury C, Paris CB, Kourafalou VH, Lirman D (2018) Dispersal capacity and genetic relatedness in Acropora cervicornis on the Florida Reef Tract. Coral Reefs 37:585–596
Drury C, Martin RE, Knapp DE, Heckler J, Levy J, Gates RD, Asner GP (2022) Ecosystem‐scale mapping of coral species and thermal tolerance. Front Ecol Environ 20(5):285–291
Edmunds PJ, Gates RD (2008) Acclimatization in tropical reef corals. Mar Ecol Prog Ser 361:307–310
Edmunds PJ, Leichter JJ, Adjeroud M (2010) Landscape-scale variation in coral recruitment in Moorea, French Polynesia. Mar Ecol Prog Ser 414:75–89
Edmunds PJ, Riegl B (2020) Urgent need for coral demography in a world where corals are disappearing. Mar Ecol Prog Ser 635:233–242
El Mahrad B, Newton A, Icely JD et al (2020) Contribution of remote sensing technologies to a holistic coastal and marine environmental management framework: A review. Remote Sens 12:2313
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Foo SA, Asner GP (2021) Impacts of remotely sensed environmental drivers on coral outplant survival. Restor Ecol 29:e13309
Forsman ZH, Ritson-Williams R, Tisthammer KH et al (2020) Host-symbiont coevolution, cryptic structure, and bleaching susceptibility, in a coral species complex (Scleractinia; Poritidae). Sci Rep 10:1–12
Fukunaga A, Kosaki RK, Pascoe KH, Burns JHR (2020) Fish assemblage structure in the Northwestern Hawaiian Islands is associated with the architectural complexity of coral-reef habitats. Diversity 12:430
Fuller ZL, Mocellin VJL, Morris LA et al (2020) Population genetics of the coral Acropora millepora: toward genomic prediction of bleaching. Science 369:eaba4674
Gilmour JP, Smith LD, Heyward AJ et al (2013) Recovery of an isolated coral reef system following severe disturbance. Science 340:69–71
Gleason DF, Hofmann DK (2011) Coral larvae: from gametes to recruits. J Exp Mar Bio Ecol 408:42–57
Glynn PW, Maté JL, Baker AC, Calderón MO (2001) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño–Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Gove JM, Williams GJ, McManus MA et al (2015) Coral reef benthic communities exhibit non-linear threshold responses to natural physical drivers. Mar Ecol Prog Ser 522:33–48
Greene A, Forsman Z, Toonen RJ, Donahue MJ (2020) CoralCam: a flexible, low-cost ecological monitoring platform. HardwareX 7:e00089
Gunderson LH (2000) Ecological resilience - In theory and application. Annu Rev Ecol Syst 31:425–439
Harrison PL (2011) Sexual reproduction of scleractinian corals. In Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 59–85
Hatcher BG (1997) Coral reef ecosystems: how much greater is the whole than the sum of the parts? Coral Reefs 16:S77–S91
Hedley JD, Roelfsema CM, Chollett I et al (2016) Remote sensing of coral reefs for monitoring and management: a review. Remote Sens 8:118
Hein MY, Beeden R, Birtles A et al (2020) Coral restoration effectiveness: Multiregional snapshots of the long-term responses of coral assemblages to restoration. Diversity 12:153
Hernandez-Agreda A, Gates RD, Ainsworth TD (2017) Defining the core microbiome in corals’ microbial soup. Trends Microbiol 25:125–140
Hernández-Landa RC, Barrera-Falcon E, Rioja-Nieto R (2020) Size-frequency distribution of coral assemblages in insular shallow reefs of the Mexican Caribbean using underwater photogrammetry. PeerJ 8:e8957
Hixon MA (2011) 60 years of coral reef fish ecology: past, present, future. Bull Mar Sci 87:727–765
Hodgson G (1999) A global assessment of human effects on coral reefs. Mar Pollut Bull 38:345–355
Hoegh-Guldberg O, Pendleton L, Kaup A (2019) People and the changing nature of coral reefs. Reg Stud Mar Sci 30:100699
Holling C (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23
Holling CS, Meffe GK (1996) Command and control and the pathology of natural resource management. Conserv Biol 10:328–337
Howells EJ, Abrego D, Meyer E et al (2016) Host adaptation and unexpected symbiont partners enable reef-building corals to tolerate extreme temperatures. Glob Chang Biol 22:2702–2714
Hughes TP (1984) Population dynamics based on individual size rather than age: a general model with a reef coral example. Am Nat 123:778–795
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hughes TP, Graham NAJ, Jackson JBC et al (2010) Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol 25:633–642
Hughes TP, Kerry JT, Álvarez-Noriega M et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Kerry JT, Baird AH et al (2018) Global warming transforms coral reef assemblages. Nature 556:492–496
Hughes TP, Kerry JT, Baird AH et al (2019) Global warming impairs stock–recruitment dynamics of corals. Nature 568:387–390
Hughes TP, Rodrigues MJ, Bellwood DR (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Hughes TP, Tanner JE (2000) Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81:2250–2263
Hume BCC, Smith EG, Ziegler M et al (2019) SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Resour 19:1063–1080
Iglesias-Prieto R, Matta JL, Robins WA, Trench RK (1992) Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc Natl Acad Sci 89:10302–10305
Innis T, Cunning R, Ritson-Williams R et al (2018) Coral color and depth drive symbiosis ecology of Montipora capitata in Kāne‘ohe Bay, O‘ahu, Hawai‘i. Coral Reefs 37:423–430
Jackson JBC (1991) Adaptation and diversity of reef corals. Bioscience 41:475–482
Jackson JBC, Kirby M, Berger W et al (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–637
Jackson JBC, Donovan MK, Cramer KL, Lam VV (2014) Status and trends of Caribbean coral reefs: 1970–2012. IUCN, Gland
Jones RJ (2008) Coral bleaching, bleaching-induced mortality, and the adaptive significance of the bleaching response. Mar Biol 154:65–80
Jouffray J-B, Wedding LM, Norström AV et al (2019) Parsing human and biophysical drivers of coral reef regimes. Proc R Soc B 286:20182544
Kenkel CD, Matz MV (2016) Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat Ecol Evol 1:1–6
Kirk NL, Howells EJ, Abrego D et al (2018) Genomic and transcriptomic signals of thermal tolerance in heat-tolerant corals (Platygyra daedalea) of the Arabian/Persian Gulf. Mol Ecol 27:5180–5194
Knowlton N, Leray M (2015) Exploring coral reefs using the tools of molecular genetics. In: Birkeland C (ed) Coral reefs in the anthropocene. Springer, Dordrecht, pp 117–132
Kwong WK, del Campo J, Mathur V et al (2019) A widespread coral-infecting apicomplexan with chlorophyll biosynthesis genes. Nature 568:103–107
Ladd MC, Burkepile DE, Shantz AA (2019) Near-term impacts of coral restoration on target species, coral reef community structure, and ecological processes. Restor Ecol 27:1166–1176
LaJeunesse TC, Parkinson JE, Gabrielson PW et al (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28:2570–2580
LaJeunesse TC, Smith R, Walther M et al (2010) Host–symbiont recombination versus natural selection in the response of coral–dinoflagellate symbioses to environmental disturbance. Proc R Soc B Biol Sci 277:2925–2934
Leinonen R, Sugawara H, Shumway M, Collaboration INSD (2010) The sequence read archive. Nucleic Acids Res 39:D19–D21
Lepczyk CA, Wedding LM, Asner GP et al (2021) Advancing Landscape and Seascape Ecology from a 2D to a 3D Science. Bioscience 71:596–608
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Levy J, Hunter C, Lukacazyk T, Franklin EC (2018) Assessing the spatial distribution of coral bleaching using small unmanned aerial systems. Coral Reefs 37:373–387
Li J, Knapp DE, Fabina NS et al (2020) A global coral reef probability map generated using convolutional neural networks. Coral Reefs 39:1805–1815
Li J, Knapp DE, Lyons M et al (2021) Automated Global Shallow Water Bathymetry Mapping Using Google Earth Engine. Remote Sens 13:1469
Little M, George EE, Arts MGI et al (2021) Three-dimensional molecular cartography of the caribbean reef-building coral Orbicella faveolata. Front Mar Sci. https://doi.org/10.3389/fmars.2021.627724
Logan CA, Dunne JP, Ryan JS et al (2021) Quantifying global potential for coral evolutionary response to climate change. Nat Clim Change. https://doi.org/10.1038/s41558-021-01037-2
Loya Y, Sakai K, Yamazato K et al (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
MacNeil MA, Connolly SR (2015) Multi-scale patterns and processes in reef fish abundance. In: Mora C (ed) Ecology of fishes on coral reefs. Cambridge University Press, Cambridge, pp 116–124
MacNeil MA, Graham NAJ, Cinner JE et al (2015) Recovery potential of the world’s coral reef fishes. Nature 520:341–344
MacNeil MA, Graham NAJ, Polunin NVC et al (2009) Hierarchical drivers of reef-fish metacommunity structure. Ecology 90:252–264
Madin JS, Connolly SR (2006) Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature 444:477–480
Martin BT, Jager T, Nisbet RM et al (2013) Predicting population dynamics from the properties of individuals: a cross-level test of dynamic energy budget theory. Am Nat 181:506–519
Matz MV, Treml EA, Aglyamova GV, Bay LK (2018) Potential and limits for rapid genetic adaptation to warming in a Great Barrier Reef coral. PLoS Genet 14:e1007220
Mcleod E, Anthony KRN, Mumby PJ et al (2019) The future of resilience-based management in coral reef ecosystems. J Environ Manage 233:291–301
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233
Muller EB, Kooijman SALM, Edmunds PJ et al (2009) Dynamic energy budgets in syntrophic symbiotic relationships between heterotrophic hosts and photoautotrophic symbionts. J Theor Biol 259:44–57
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101
Nash KL, Graham NAJ, Jennings S et al (2016) Herbivore cross-scale redundancy supports response diversity and promotes coral reef resilience. J Appl Ecol 53:646–655
National Academies of Sciences, Engineering, and Medicine (2019a) A decision framework for interventions to increase the persistance and resilience of coral reefs. National Academies of Sciences, Engineering, and Medicine, Washington DC
National Academies of Sciences, Engineering and Medicine (2019b) A research review of interventions to increase the persistence and resilience of coral reefs. National Academies of Sciences, Engineering, and Medicine, Washington DC
Naugle MS, Oliver TA, Barshis DJ et al(2021) Variation in Coral Thermotolerance Across a Pollution Gradient Erodes as Coral Symbionts Shift to More Heat-Tolerant Genera.Front Mar Sci1670
Nyström M, Folke C (2001) Spatial resilience of coral reefs. Ecosystems 4:406–417
Nyström M, Graham N, Lokrantz J, Norström A (2008) Capturing the cornerstones of coral reef resilience: Linking theory to practice. Coral Reefs 27:795–809
Obura DO, Appeltans W, Amornthammarong N et al (2019) Coral reef monitoring, reef assessment technologies, and ecosystem-based management. Front Mar Sci 6:580
Palandro DA, Andréfouët S, Hu C et al (2008) Quantification of two decades of shallow-water coral reef habitat decline in the Florida Keys National Marine Sanctuary using Landsat data (1984–2002). Remote Sens Environ 112:3388–3399
Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanisms of reef coral resistance to future climate change. Science 344:895–898
Pandolfi J, Bradbury R, Sala E (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Paulay G (1997) Diversity and distribution of reef organisms. In: Birkeland C (ed) Life death coral reefs. Chapman and Hall, New York, pp 298–353
Peters DPC, Bestelmeyer BT, Turner MG (2007) Cross–scale interactions and changing pattern–process relationships: consequences for system dynamics. Ecosystems 10:790–796
Peterson G, Allen CR, Holling CS (1998) Ecological resilience, biodiversity, and scale. Ecosystems 1:6–18
Polato NR, Altman NS, Baums IB (2013) Variation in the transcriptional response of threatened coral larvae to elevated temperatures. Mol Ecol 22:1366–1382
Pratchett MS, Munday P, Wilson SK et al (2008) Effects of climate-induced coral bleaching on coral-reef fishes. Ecol Econ consequences Oceanogr Mar Biol Annu Rev 46:251–296
Putnam HM, Davidson JM, Gates RD (2016) Ocean acidification influences host DNA methylation and phenotypic plasticity in environmentally susceptible corals. Evol Appl 9:1165–1178
Ritson-Williams R, Arnold SN, Fogarty ND et al (2009) New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson Contrib Mar Sci 38:437
Ritson-Williams R, Arnold SN, Paul VJ (2016) Patterns of larval settlement preferences and post–settlement survival for seven Caribbean corals. Mar Ecol Prog Ser 548:127–138
Ritson-Williams R, Gates RD (2020) Coral community resilience to successive years of bleaching in Kāne ‘ohe Bay, Hawai ‘i. Coral Reefs 39:757–769
Roach TNF, Dilworth J, Jones AD et al (2021) Metabolomic signatures of coral bleaching history. Nat Ecol Evol 5:495–503
Roche RC, Williams GJ, Turner JR (2018) Towards developing a mechanistic understanding of coral reef resilience to thermal stress across multiple scales.Curr Clim Chang Rep 4:51–64
Rosado PM, Leite DCA, Duarte GAS et al (2019) Marine probiotics: increasing coral resistance to bleaching through microbiome manipulation. ISME J 13:921–936
Rose NH, Bay RA, Morikawa MK, Palumbi SR (2018) Polygenic evolution drives species divergence and climate adaptation in corals. Evolution (N Y) 72:82–94
Roth L, Koksal S, Van Woesik R (2010) Effects of thermal stress on key processes driving coral-population dynamics. Mar Ecol Prog Ser 411:73–87
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Rowan R, Powers DA (1992) Ribosomal RNA sequences and the diversity of symbiotic dinoflagellates (zooxanthellae). Proc Natl Acad Sci USA 89:3639–3643
Safaie A, Silbiger NJ, McClanahan TR et al (2018) High frequency temperature variability reduces the risk of coral bleaching. Nat Commun 9:1671
Saha K, Zhao X, Zhang H et al (2018) The Coral Reef Temperature Anomaly Database (CoRTAD) Version 6—Global, 4 km Sea Surface Temperature and Related Thermal Stress Metrics for 1982 to 2019. NOAA National Centers for Environmental Information
Salameh E, Frappart F, Almar R, Baptista P, Heygster G, Lubac B, Raucoules D, Almeida LP, Bergsma EW, Capo S, De Michele M (2019) Monitoring beach topography and nearshore bathymetry using spaceborne remote sensing: A review. Remote Sensing 11(19):2212
Savary R, Barshis DJ, Voolstra CR et al (2021) Fast and pervasive transcriptomic resilience and acclimation of extremely heat-tolerant coral holobionts from the northern Red Sea. Proc Natl Acad Sci USA 118(19):e2023298118
Schill SR, Asner GP, McNulty VP et al (2021) Site selection for coral reef restoration using airborne imaging spectroscopy. Front Mar Sci. https://doi.org/10.3389/fmars.2021.698004
Selkoe KA, Gaggiotti OE, Treml EA et al (2016) The DNA of coral reef biodiversity: predicting and protecting genetic diversity of reef assemblages. Proc R Soc B R Soc 283:20160354
Seneca FO, Palumbi SR (2015) The role of transcriptome resilience in resistance of corals to bleaching. Mol Ecol 24:1467–1484
Steneck R, Arnold SN, Boenish R et al (2019) Managing recovery resilience in coral reefs against climate-induced bleaching and hurricanes: a 15 year case study from Bonaire, Dutch Caribbean. Front Mar Sci 6:265
Steneck RS, Mumby PJ, MacDonald C et al (2018) Attenuating effects of ecosystem management on coral reefs. Sci Adv 4:eaao5493
Stuart-Smith RD, Brown CJ, Ceccarelli DM, Edgar GJ (2018) Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560:92
Stuart-Smith RD, Edgar GJ, Barrett NS et al (2017) Assessing national biodiversity trends for rocky and coral reefs through the integration of citizen science and scientific monitoring programs. Bioscience 67:134–146
Toonen RJ, Andrews KR, Baums IB et al (2011) Defining boundaries for ecosystem-based management: a multispecies case study of marine connectivity across the Hawaiian Archipelago. J Mar Biol 2011:1–13
Trevathan J, Johnstone R, Chiffings T et al (2012) SEMAT—the next generation of inexpensive marine environmental monitoring and measurement systems. Sensors 12:9711–9748
Turner MG, Gardner RH, O’Neill RV (2001) Landscape ecology in theory and practice. Springer, New York
Underwood JN, Richards ZT, Miller KJ et al (2018) Genetic signatures through space, time and multiple disturbances in a ubiquitous brooding coral. Mol Ecol 27:1586–1602
Van Oppen MJH, Oliver JK, Putnam HM, Gates RD (2015) Building coral reef resilience through assisted evolution. Proc Natl Acad Sci USA 112:2307–2313
van Woesik R, Sakai K, Ganase A, Loya Y (2011) Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser 434:67–76
Vega Thurber R, Payet JP, Thurber AR, Correa AMS (2017) Virus–host interactions and their roles in coral reef health and disease. Nat Rev Microbiol 15:205–216
Vega Thurber R, Willner-Hall D, Rodriguez‐Mueller B et al (2009) Metagenomic analysis of stressed coral holobionts. Environ Microbiol 11:2148–2163
Vollmer SV, Kline DI (2008) Natural disease resistance in threatened staghorn corals. PLoS ONE 3:e3718
Voolstra CR, Miller DJ, Ragan MA et al (2015) The ReFuGe 2020 Consortium—using “omics” approaches to explore the adaptability and resilience of coral holobionts to environmental change. Front Mar Sci 2:68
Warwick RM, Clarke KR (1990) A statistical analysis of coral community responses to the 1982–83 El Niño in the Thousand Islands, Indonesia. Coral Reefs 8:171–179
Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3:385–397
Williams G, Gove J, Eynaud Y et al (2015) Local human impacts decouple natural biophysical relationships on Pacific coral reefs. Ecography (Cop) 38:1–11
Williams GJ, Knapp IS, Maragos JE, Davy SK (2010) Modeling patterns of coral bleaching at a remote Central Pacific atoll. Mar Pollut Bull 60:1467–1476
Williams ID, Couch CS, Beijbom O et al (2019) Leveraging automated image analysis tools to transform our capacity to assess status and trends of coral reefs. Front Mar Sci 6:222
Ziegler M, Seneca FO, Yum LK et al (2017) Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat Commun 8:14213
Acknowledgements
The ideas and perspectives for this work were generated at a working group at the National Center for Ecological Analysis and Synthesis for the Coral Reef Science and Cyberinfrastructure Network (CRESCYNT). We acknowledge Julian Brun, Jeanette Clark, Ken Johnson, David Knapp, Eric Lingerfelt, Robin Martin, Robert McGuinn, and Julia Stewert-Lowndes for participating in discussions that led to this work. CRESCYNT is a Research Coordination Network supported by the National Science Foundation under Grant No. 1440342. A portion of this study was supported by a Lenfest Ocean Program grant to G. Asner, and by Paul G. Allen Family Foundation and CZI grants to C. Drury. The Global Airborne Observatory is made possible by support provided by private foundations, visionary individuals, and Arizona State University.
Funding
National Science Foundation under Grant No. 1440342, Lenfest Ocean Program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and review of this perspective. Figures were generated by M. Donovan, J. Burns, and C. Drury. Case study analyses were performed by C. Drury.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donovan, M.K., Alves, C., Burns, J. et al. From polyps to pixels: understanding coral reef resilience to local and global change across scales. Landsc Ecol 38, 737–752 (2023). https://doi.org/10.1007/s10980-022-01463-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01463-3