Abstract
Context
Climate and land-use changes affect species ranges and movements. However, these changes are usually overlooked in connectivity studies, and this could have adverse consequences in the definition of effective management measures.
Objectives
We evaluated two ways to incorporate landscape dynamics: (i) by analyzing connectivity as a fluctuating phenomenon (i.e., time-varying connectivity); and (ii) by analyzing species movements from past to current ranges (i.e., spatio-temporal connectivity). We also compared these dynamic approaches with traditional static connectivity methods.
Methods
We compared the overall connectivity values and the prioritization of critical habitat patches according to dynamic and static approaches using habitat availability metrics (Probability of Connectivity and Equivalent Connected Area). This comparative research was conducted for species associated with broadleaf forests of the different ecoregions of the Iberian Peninsula. We considered species habitat preferences during movement and a wide range of dispersal abilities to assess functional connectivity.
Results
Static approaches generated varying overall connectivity values and priority patches depending on the time snapshot considered and different from those generated by dynamic approaches. The two dynamic connectivity approaches resulted in very similar priority conservation patches, indicating their potential to guide enduring conservation measures that enhance connectivity between contemporary habitat patches at multiple time snapshots but also species range shifts in time.
Conclusions
Connectivity is affected by landscape changes, and only dynamic approaches can overcome the issues associated with these changes and provide valuable information to guide improved and enduring measures in changing landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Landscapes change due to the natural habitat dynamics, but also due to climate (Mora et al. 2013) and land-use variations (Song et al. 2018). These changes may affect vegetation distribution and structure (Root et al. 2003; Beltrán et al. 2014), water availability (Taylor et al. 2013; Bishop-Taylor et al. 2018a), and other factors affecting species habitats. Changes in habitat distribution, amount, and quality may result in species’ range shifts (among other responses such as phenological, behavioral, or genetic adaptation) to ensure species persistence (Parmesan and Yohe 2003; Chen et al. 2011; Davidson et al. 2020; Román-Palacios and Wiens 2020). However, habitat fragmentation can complicate and even jeopardize species colonization of new areas with suitable habitat (Collinge 1996; Honnay et al. 2002). The enhancement of habitat connectivity is considered a key strategy to mitigate the detrimental effects of habitat fragmentation, as it eases the flow of resources, species, genes, and ecological processes through the landscape (Correa Ayram et al. 2015; Keeley et al. 2018a).
Changes in the spatial configuration, amount, and quality of species habitats, as well as the matrix in-between habitat patches, may alter the flow of species and ecological processes, involving changes in connectivity over time. Therefore, connectivity is increasingly recognized as a time-varying phenomenon (Keeley et al. 2018b; Zeller et al. 2020). However, connectivity has traditionally been studied using a static and immutable approach, which identifies landscape areas that promote the exchange of individuals and processes between contemporary habitat patches at a unique current snapshot (Nuñez et al. 2013; Albert et al. 2017; Costanza et al. 2020). These static approaches and conservation measures might not be appropriate at another time. Connectivity changes over time (time-varying connectivity) have only recently been incorporated into connectivity studies by analyzing landscapes at multiple time snapshots (Saura et al. 2011; Beltrán et al. 2014; Bishop-Taylor et al. 2018b; Jennings et al. 2020). These studies assess and combine multiple static connectivity approaches considering the connections among contemporary patches for each snapshot. They usually identify stable areas (i.e., habitat patches or corridors) that persist with suitable conditions for the focal species over time as targets for conservation measures. These stable areas (the so-called climate refugia in the case of climate change) are defined by aggregating the results of the multiple snapshots. Time- varying connectivity studies have corroborated that connectivity is a dynamic phenomenon and does not remain constant over time. Considering connectivity as a time-varying phenomenon is important to adequately guide long-term management measures that enhance functional connectivity over a broad period of time.
So far, dynamic connectivity studies have mainly considered changes in connectivity over time (i.e., time-varying connectivity) but rarely considered whether landscape features would allow the shift of species at a specific snapshot to their future suitable ranges (Williams et al. 2005; Phillips et al. 2008; Lawler et al. 2013; Cushman 2015; Littlefield et al. 2017; Martensen et al. 2017; Carroll et al. 2018; Ash et al. 2020; Gray et al. 2020; Huang et al. 2020; Zhao et al. 2021). However, the distribution of suitable habitat and the surrounding landscape change, and the pattern of these changes may become a key factor to enable organisms to reach their future potential distribution areas and keep up with landscape changes, especially for poor dispersers (Williams et al. 2005). Spatio-temporal connectivity approaches assess species shifts to their future suitable areas by considering the connectivity from past to future snapshots. Thus, spatio-temporal analyses might be a step forward to allow more realistic, robust, and ecologically meaningful connectivity assessments than traditional static or time-varying analyses. The scarce work reported on this topic was also greatly simplified as most studies did not account for particularities of the dynamics, the focal species, and/or the landscape. For instance, most of them did not consider network directionality (Acevedo et al. 2015), i.e., flow can only occur from past to future snapshots, and not in the opposite direction. Another usual simplification was connecting climate refugia. This implied considering climate as the only important factor in species habitat selection, although other variables such as anthropic pressure or land cover are known to be of the most importance in habitat selection (Mateo Sánchez et al. 2014; Gastón et al. 2019; Banfield et al. 2020; Parks et al. 2020). Other spatio-temporal connectivity assessments studied the connections between static protected areas; however, these areas might change (Elsen et al. 2020) and stop being suitable for species in the future. Furthermore, very few functional connectivity studies have accounted for the landscape dynamics and, at the same time, for species dispersal abilities and habitat preferences for movement (Keeley et al. 2018b; Zeller et al. 2020). In fact, most dynamic studies have disregarded the state and changes in the landscape matrix and how it impacts species movements, that is, the landscape resistance (Spear et al. 2010; Zeller et al. 2012; Mateo-Sánchez et al. 2015; Keeley et al. 2016). Integrating all these particularities would lead to an improved evaluation of spatio-temporal connectivity to comprehensively guide sound and feasible decisions for landscape management.
Importantly, and due to the limited resources allocated to conservation actions, it is essential to identify and focus efforts and means on the most critical landscape areas. A few time-varying and spatio-temporal connectivity studies prioritized conservation areas (Albert et al. 2017; Bishop-Taylor et al. 2018b; Jennings et al. 2020; Conlisk et al. 2021; Zhao et al. 2021). These studies mainly accounted for the permanence over time of quality areas or their spatial probability of connection (i.e., effective distance). However, apart from these characteristics, it is important to regard the irreplaceability (Wilson et al. 2009) of the areas. In other words, consider whether alternative landscape elements could, at least partially, supplant the contribution to the overall connectivity of the considered area in case it gets lost or degraded. Some graph-based (Urban et al. 2009) habitat availability metrics were proposed to include irreplaceability in connectivity prioritization: the Probability of Connectivity (PC) (Saura and Pascual-Hortal 2007; Saura and Rubio 2010), and the Equivalent Connected Area (ECA) (Saura et al. 2011; Santini et al. 2016). These indices proved to be good indicators to monitor landscape changes (Bishop-Taylor et al. 2018a; Poli et al. 2019; Keeley et al. 2021) and very useful for quantifying overall connectivity and identifying critical elements to maintain or enhance it. However, so far, they have been mainly used under a static approach (Dondina et al. 2017; de la Fuente et al. 2018; Cisneros-Araujo et al. 2021) despite their potential to be adapted to different spatial and temporal probabilities of connectivity and to deal with the directionality of the links. Martensen et al. (2017) adapted these metrics to be applied in spatio-temporal connectivity studies. These newly customized metrics can be used to calculate the reachable habitat through spatial and temporal connections. Yet, these metrics have only been applied to calculate overall dynamic connectivity levels (Huang et al. 2020) and patches Betweenness Centrality (Zhao et al. 2021), which measures patch importance (Bodin and Norberg 2007) but not its irreplaceability to provide ecological flows.
Our goal here was to conduct a quantitative and qualitative comparative study on the implications of three connectivity approaches: (i) static, (ii) time-varying, and (iii) spatio-temporal. Specifically, we compared the overall connectivity and priority patches obtained from these three approaches in the period from 1990 (initial snapshot, t1) to 2018 (final snapshot, t2). To do so, we focused on broadleaf forests along the different ecoregions of the Iberian Peninsula. We followed a functional approach (i.e., a resistance-based approach that accounts for species habitat selection) (Tischendorf and Fahrig 2000) in a multispecies framework (i.e., we considered a wide range of dispersal distances to capture the variable dispersal abilities of the species associated with the focal habitat). Therefore, this study also evaluated the change in connectivity (a) for species with different vagility (Costanza et al. 2020) and (b) among ecoregions with different ecological and climatic characteristics and trends (Olson et al. 2001). As far as we are aware, this study is the first analysis of time-varying and spatio-temporal connectivity that includes the mentioned particularities of the landscape dynamics, the focal species, and the landscape matrix and prioritizes habitat patches by their contribution (or importance) and irreplaceability. Moreover, this work contributes to a more comprehensive understanding of the implications of incorporating dynamics in connectivity studies to guide more efficient and informed conservation planning.
Materials and methods
Study area, ecoregions, and species
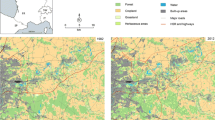
This study was carried out on the Iberian Peninsula (Fig. 1), South-Western Europe, an area especially affected by landscape changes (Loarie et al. 2009; Song et al. 2018). It has an extent of 581,000 km2 and is comprised of two countries, Spain and Portugal. It is a large and heterogeneous area that encompasses very different ecological characteristics, vegetation communities, and habitat types which determine flora and fauna distribution. To account for species habitat delimitations, we used the terrestrial biogeographic units or ecoregions delineated by Olson et al. (2001), which reflect the distribution of a broad range of species and communities across the Earth. We assumed that species remain in the same ecoregion and only roam between habitat patches (i.e., broadleaf forests) with similar ecological conditions (i.e., belonging to the same ecoregion).
Ecoregions of the Iberian Peninsula and their relative habitat coverage (i.e., percentage ecoregion area covered by broadleaf forest) in 1990. The ecoregions names can be found in Table 1. Coordinate system ETRS 1989 UTM Zone 30 N
The diversity of focal species (i.e., broadleaf forest specialists) was represented by a wide range of median dispersal distances: 1 km, 2 km, 5 km, 10 km, 30 km, and 50 km. These distances condition species arrival to destination habitat patches and capture most dispersal abilities of terrestrial animals (Sutherland et al. 2000; Santini et al. 2013) from short (e.g., Rana dalmantina, Lacerta bilineata, Garrulus glandarius, Meles meles, etc.) through medium (e.g., Asio otus, Tetrao urogallus, Capreolus capreolus, Accipiter gentilis, Cervus elaphus, Sus scrofa, Felis silvestris, Genetta genetta, Martes martes, etc.) to long-distance (e.g., Ursus arctos, Lynx pardinus, Canis lupus, Aquila adalberti, etc.) dispersers. Moreover, large mean dispersal distances may also depict unusual long movements of shorter-distance dispersers.
Methods
We compared three different approaches to study connectivity: (i) static; (ii) time-varying; and (iii) spatio-temporal. The static approach (i.e., considering connections between contemporary habitat patches at a single time snapshot) was followed twice independently, one at an initial time snapshot t1 (1990) and another one at a final snapshot t2 (2018). These two static runs were denoted by s1 and s2 respectively. Time-varying and spatio-temporal approaches represent two different ways to incorporate dynamics in connectivity analyses. The time-varying approach (denoted by tv) considered together both time snapshots (t1 and t2) just by combining the results of the two static runs and reflected how connectivity had changed over time. Lastly, the spatio-temporal approach (denoted by st) reflected the connection of habitat patches at t1 with those at t2. All analyses were developed separately for each ecoregion and dispersal distance. Figure 2 summarizes the workflow followed.
Methodological workflow scheme. 1. We defined three different sets of landscape conditions (section “Landscape conditions definition: network and matrix characteristics”): static at t1 (1990), static at t2 (2018), and spatio-temporal from t1 to t2. To do so, we (a) identified the habitat patches (section “Definition of habitat patches and nodes”), (b) calculated the resistance surface (section “Landscape matrix characterization: resistance surface”), (c) determined the connections between patches with least-cost path modeling and their spatial probability of connection (section “Connections between nodes: spatial probability of dispersal”), and (d) calculated the temporal probability of the connections for the spatio-temporal landscape conditions (section “Spatio-temporal adjustment of connections: temporal probability of dispersal”). 2. Connectivity analyses (section “Connectivity analyses”): we calculated different connectivity metrics (Table 3) to measure: (a) the overall connectivity (section “Overall connectivity”) and (b) the individual node importance to ultimately identify priority conservation patches (section “Identification of priority habitat patches”). This second stage was conducted for the three landscape conditions and six dispersal distances. 3. Comparison of connectivity approaches: we compared the connectivity results of each approach (static, time-varying, or spatio-temporal approach)
Landscape conditions definition: network and matrix characteristics
We established three different sets (two static and one spatio-temporal) of landscape conditions (i.e., the combination of habitat network and matrix characteristics) to represent the landscape for the different connectivity approaches. These landscape conditions encompassed: (a) the number, distribution, and area of focal habitat patches; (b) the characteristics of the surrounding matrix; (c) the spatial probability of connectivity between patches; and (d) the temporal probability of connectivity between spatio-temporal patches. These characteristics varied from t1 to t2, and therefore each run of the static approach (s1 and s2), as well as the spatio-temporal approach, had different landscape conditions associated. However, the time-varying approach did not require to define additional landscape conditions as it only combines the two static approaches, with their respective landscape conditions.
Definition of habitat patches and nodes
First, habitat cells were determined as pixels covered by broadleaf forest at t1, t2, or both time snapshots according to CORINE land cover maps (100-m resolution) from 1990 and 2018. Subsequently, these habitat cells were classified as (a) ‘stable’ when identified as habitat in both time snapshots; (b) ‘lost’ if they were identified only at t1, and (c) ‘gained’ when identified only at t2. We then delineated habitat patches as contiguous habitat cells with the same temporal classification (stable, lost, or gained) with a surface over 50 ha. This large patch size threshold was set for two reasons: first, to ensure that patches can host individuals throughout their entire lifetime to overcome species connectivity over generations (Keeley et al. 2018a), especially for large dispersers, and second, for feasible computational processing. The centroid of these habitat patches defined the network nodes (Urban et al. 2009), which were qualified by their area (i.e., patch attribute) as an estimation of species abundance (Drake et al. 2021). We only used stable and lost nodes for the initial static landscape conditions, while stable and gained nodes for the final static landscape conditions (Fig. 3). The spatio-temporal approach connects nodes at t1 with those at t2, and therefore, we used the whole set of nodes (but differentiating stable, lost, and gained nodes) under the spatio-temporal landscape conditions. We delineated and characterized habitat patches and nodes with the ArcGIS software.
Representation of the habitat patches and connections between them for the three landscape conditions: A static at t1 (1990); B static at t2 (2018); and C spatio-temporal from t1 to t2. Stable (active at t1 and t2), lost (active at t1), and gained (active at t2) patches are represented as green, blue, and yellow shapes respectively. One and two-way arrows represent the possible links and the direction of the connection between nodes. All the connections in the static landscape conditions (A and B) are bidirectional, while they are directed in the spatio-temporal landscape conditions (C), only connecting nodes active at t1 (stable and lost) with nodes active at t2 (stable and gained). Solid and dashed arrows in the spatio-temporal landscape conditions denote the temporal probability of 1 and 0.5 respectively according to nodes' coexistence
Landscape matrix characterization: resistance surface
Next, the landscape matrix was characterized for each set of landscape conditions through resistance surfaces (Tischendorf and Fahrig 2000; Spear et al. 2010; Zeller et al. 2012) with the ArcGIS software. These surfaces depicted how the landscape allowed or impeded species movement. To create them, we assigned different resistance values to each land cover class/use according to Table S1 (in the supplementary data) based on previous studies of forest species in Spain (Gurrutxaga et al. 2011; Ruiz-González et al. 2014; de la Fuente et al. 2018) and expert opinion. These resistance values ranged from 1 to 1000, with increasing values corresponding to classes that restrain forest species movement. Resistance surfaces for the initial and final static landscape conditions were created from CORINE land cover maps at t1 and t2 respectively and combined with the distribution of roads from OpenStreetMap (www.openstreetmap.org) to include the influence of linear infrastructures on species movements (Banfield et al. 2020). On the other hand, as the movement from a patch at t1 to a patch at t2 (in the spatio-temporal approach) could be at any time in-between t1 and t2, and we did not have information on the resistance at that precise moment, we defined the resistance surface of the spatio-temporal landscape conditions as the mean of the two static surfaces. All resistance surfaces were resampled at a spatial resolution of 200 m to reduce execution times by aggregating the cells with a factor of 2 and the mean function.
Connections between nodes: spatial probability of dispersal
We applied least-cost path modeling (Adriaensen et al. 2003) to determine the connections among nodes with the R package gDistance. For each set of landscape conditions, we connected each node with all the nodes of the same ecoregion. The effective distance of these connections (i.e., the accumulated resistance along the least-cost path) depicted the difficulty of moving between each pair of nodes through the resistance surface. These effective distances were then converted into probabilities of direct dispersal between each pair of nodes (i.e., spatial probability of dispersal) according to the spatial configuration of habitat patches, the resistance surface, and the dispersal capacity of the species. This conversion was estimated with a negative exponential function of the effective distance between patches (Urban and Keitt 2001; Saura and Pascual-Hortal 2007; Gurrutxaga et al. 2011; Saura et al. 2017, 2018). The steepness of this function was fitted to the dispersal abilities of the focal species by assigning a probability of 0.5 to a distance equal to the species’ median effective dispersal distance (Online Appendix S1). To estimate the species’ median effective dispersal distance, we multiplied the previously defined lineal dispersal distances (i.e., 1, 2, 5, 10, 30, and 50-km) by the median landscape resistance value of the spatio-temporal landscape conditions (Gurrutxaga and Saura 2013). Finally, we excluded connections with a spatial probability of connectivity below 0.001 and obtained a set of viable connections between nodes with their spatial probability of connection for each set of landscape conditions and dispersal distance. We used R software for the calculations.
Spatio-temporal adjustment of connections: temporal probability of dispersal
In the spatio-temporal landscape conditions, aside from the spatial probability of dispersal, we incorporated a temporal component (i.e., the temporal probability of dispersal between nodes) (Martensen et al. 2017). On the one hand, this temporal component (Fig. 3 and Table 2) dealt with the directionality of the network: successful direct spatio-temporal connectivity only occurred with the connection of patches active at an initial time t1 with patches active at a later final time t2. These links only existed in this direction, as it is impossible to move from future to past patches. Therefore, links that started at a gain node or ended at a loss node were associated with a null temporal probability of connectivity. On the other hand, the temporal component also depicted nodes’ coexistence: species could only move between contemporary patches that are active simultaneously at any time between t1 and t2. In this respect, links starting and/or ending at stable nodes had an associated temporal probability of 1, as the starting and ending nodes were sure to coexist during a certain period. Finally, links starting at a lost node and finishing at a gain node should have intermediate temporal probabilities of connectivity (between 0 and 1) depending on the coexistence period. However, we did not examine the actual length of the overlapping period of each of these links and associated a temporal probability of 0.5 (14 years of coexistence) to all of them. To incorporate this temporal component in the spatio-temporal landscape conditions, we multiplied the spatial probability by the temporal probability of connectivity of each link. It resulted in the spatio-temporal probability that an individual moves from a given node at t1 to another node at t2.
Connectivity analyses
We performed a comparative assessment of the analyzed connectivity approaches (static, time-varying, and spatio-temporal) and the different ecoregions and dispersal distances. To do so, we calculated and contrasted (i) the overall connectivity of the network (section “Overall connectivity”), and (ii) the key habitat patches to maintain the overall connectivity (section “Identification of priority habitat patches”) using graph-based habitat availability metrics (Pascual-Hortal and Saura 2006; Saura and Pascual-Hortal 2007; Saura and Rubio 2010). All connectivity metrics were calculated with the command line Conefor software (Saura and Torné 2009, www.conefor.org). The spatio-temporal landscape conditions required the version for directed networks.
Overall connectivity
We used the Equivalent Connected Area (ECA, see Table 3 and Saura et al. 2011; Santini et al. 2016) to determine the overall level of connectivity of each ecoregion, set of landscape conditions, and dispersal distance. We calculated the ECA for the two static landscape conditions (ECA1 at t1 and ECA2 at t2), while for the spatio-temporal one we used a dynamic adaptation of this metric (ECAst) (Martensen et al. 2017), best suited for the particularities of these landscape conditions. The ECA of the entire study area was calculated by summing the ECA of every ecoregion. Afterward, we calculated the connectivity change (dECA, Table 3): (a) from the initial to the final static landscape conditions (dECAtv) to measure the changes in connectivity over time (time-varying connectivity); and (b) from the initial to the spatio-temporal landscape conditions (dECAst) to measure the difference in connectivity between following a static and a spatio-temporal approach. ECA and dECA metrics have area units and thus can be easily interpreted and compared with the existing focal habitat area and its variation dA (Table 3) (Saura et al. 2011).
Identification of priority habitat patches
To characterize patch importance, we used the relative variation in the metric PC (dPC, Table 3) when systematically removing each habitat patch (Saura and Pascual-Hortal 2007). The patches with the highest dPC are the most important and irreplaceable to maintain the overall landscape connectivity, and therefore should be considered as critical and priority elements. To facilitate comparability between ecoregions and approaches and account for the diversity of species associated with the focal habitat, we integrated the patch importance of all dispersal distances considered. For this purpose, we min–max normalized and summed the individual importance of all dispersal distances and obtained the generalized relative importance for each patch and set of landscape conditions (dPC1, dPC2, and dPCst for the static at t1, static at t2, and spatio-temporal landscape conditions respectively). We used separately dPC1 and dPC2 to inform about patches’ importance in the two static approaches, however, we summed these two metrics to measure the time-varying connectivity importance (dPCtv).
Finally, to select the priority patches for each ecoregion and approach, we identified the patches with the highest generalized relative importance whose area sum less than 10% of the total ecoregion area. To ensure comparability between the three connectivity approaches, we selected two sets of priority patches for each of the two dynamic approaches (time-varying and spatio-temporal), one including only patches active at t1, and another one with patches active at t2. In this way, six sets of priority patches were created (Prior1, Prior 2, Priortv_1, Priortv_2, Prior st_1, and Priorst_2). We then calculated the percentage of priority area shared by the six sets of priority patches to compare the prioritization through the different connectivity approaches. These percentages of shared priority area were calculated for each ecoregion and for the entire study area by considering together the priority areas of every ecoregion.
Results
Changes in the topology of the habitat network over time.
There was a general increase in broadleaf forest area (dA) in the Iberian Peninsula of 40.11% (Table S2, Fig. 4), covering 11 and 15% of the study area in 1990 and 2018 respectively. Most of the habitat was classified as gained (absent at t1 and present at t2), followed by stable and lost (Fig. 4). Most ecoregions experienced an increase in total habitat area and mean patch size (Table S2). Only ecoregion 21 (Southwest Iberian Mediterranean sclerophyllous and mixed forests) showed a decrease in its total habitat area and mean patch size. The ecoregion 19 (Southeastern Iberian shrubs and woodlands) did not have any habitat patch in 1990 and only a small patch in 2018, therefore we did not present the results of this ecoregion. Inland Iberian ecoregions (Iberian sclerophyllous and semi-deciduous forests, and Iberian conifer forests ecoregions, i.e., ecoregions 9 and 8) had the smallest relative habitat area (i.e., the proportion of area covered by habitat) and the smallest mean habitat patch size at both time snapshots. Despite the total increase in habitat, there was a degradation of the surrounding landscape matrix for the movement of focal species, indicated by an increase in the mean resistance of the study area from 72.32 to 81.28.
Static connectivity
As expected, overall connectivity (ECA) increased with the dispersal abilities of the species (Fig. 5) for both s1 and s2 static landscape conditions. This growth in connectivity is more notable from low to medium dispersal distances (from 1 to 20 km). Priority patches drastically differed between static landscape conditions (Table S3, Fig. s4). In fact, only 35.9% of the final priority patches (at t2) of the entire study area were identified as a priority in the initial landscape conditions (at t1). This percentage of shared priority habitat area between the two static landscape conditions varied between ecoregions from 0 to 60.7% in Cantabrian mixed forests (ecoregion 6), and Southwest Iberian Mediterranean sclerophyllous and mixed forests (ecoregion 21) respectively.
Time-varying connectivity
The overall connectivity (ECA) showed a mean increase of 39.57% (standard deviation of 1.2) from 1990 to 2018 for all dispersal distances (Fig. 5). This connectivity change (dECAtv) remained positive and stable across all dispersal distances, and with a very similar magnitude to the habitat area change (dA) when considering the whole study area (Fig. S1). However, most ecoregions showed a higher increase in overall connectivity for species with low dispersal capacity (Fig. S2), indicating that vagile species are less sensitive to changes in connectivity. In fact, in most ecoregions, this increase in overall connectivity was greater than the increase in total habitat area for short dispersal distances, while it was similar or smaller for large dispersal distances (Fig. S3). On the contrary, ecoregions 6 (Cantabrian mixed forests), 8 (Iberian conifer forests), and 21 (Southwest Iberian Mediterranean sclerophyllous and mixed forests) showed a larger increase in habitat area than in overall connectivity. Particularly, this last ecoregion was the only region that experienced a decrease in habitat connectivity. The priority areas identified when following a time-varying approach covered a mean of 99.8 and 98.7% of the priority area from the initial and final static landscape conditions respectively (Table S3 and Fig. S4).
Spatio-temporal connectivity
The overall connectivity for the spatio-temporal landscape conditions (ECAst) was in an intermediate state between the two static landscape conditions for all dispersal distances (Fig. 5). As in the static landscape conditions, the spatio-temporal overall connectivity increased with the dispersal distance for species with limited dispersal capabilities and remained almost stable for medium and large dispersal distances. Generally, there was a positive connectivity change (dECAst) (Fig. S1) that highlights the connectivity gain from t1 to t2. Most ecoregions also followed this trend (Fig. S5), yet ecoregions 8, 16, and 21 (Iberian conifer forests, Northwest Iberian montane forests, and Southwest Iberian Mediterranean sclerophyllous and mixed forests) had less connectivity in the spatio-temporal landscape conditions than in the initial static landscape conditions, as shown by the negative value of the connectivity change. This last ecoregion experienced a loss of both habitat and connectivity, and thus this negative change in connectivity was expected. However, ecoregions 8 and 16 presented an increase in habitat and connectivity from 1990 to 2018 when not considering the spatio-temporal dynamics.
Priority nodes identified in the spatio-temporal landscape conditions matched closely with those from the static landscape conditions (Table S3 and Fig. S4 in the supplementary data). A mean of 98.9 and 97.9% of the priority patches in the initial and final static landscape conditions were also detected as priority areas in the spatio-temporal landscape conditions. Therefore, the results of spatio-temporal connectivity identified priority conservation areas to ensure species range shifts, but also the maintenance of connectivity in specific snapshots. Additionally, 97% of the time-varying priority area was also identified as a priority when following the spatio-temporal approach. That is, the resulting key conservation areas were almost the same in the two dynamic approaches.
Discussion
Overall implications
The abundant evidence of climate (Mora et al. 2013) and land-use changes (Song et al. 2018), and their influence on species distribution (Parmesan and Yohe 2003; Chen et al. 2011; Davidson et al. 2020) highlight the need to incorporate landscape dynamics in conservation studies. Understanding how these landscape changes influence species connectivity is an important step forward in contemporary connectivity research and conservation planning. This work aimed to gain insights into the use of habitat availability indices (Saura and Pascual-Hortal 2007; Saura and Rubio 2010) under two different dynamic approaches and to assess potential conceptual and practical differences among them and with traditional static approaches. Furthermore, the variety of considered ecoregions and species (i.e., dispersal abilities) allow a comprehensive interpretation of the results to advance in the incorporation of functional connectivity concerns for a wide array of species in changing landscapes. Therefore, this study may set a practical example to guide other analyses within a different management context (i.e., different study areas, focal species, landscape changes, and time snapshots). Particularly, the framework presented here may be applied to future projections, which would allow conservationists to anticipate future landscape changes and to implement improved measures with more functional and enduring conservation results.
Changes in the topology of the habitat network over time
We found a generalized increase in the broadleaf forest area in the Iberian Peninsula. This growth is consistent with other studies that found a net forest gain in the last years in Europe, and specifically in Spain and Portugal (FAO 2015), and all the biomes found in the Iberian Peninsula (Song et al. 2018), but especially in mountainous regions (ecoregions 33, 16, and 8) and temperate oceanic regions (ecoregion 6). However, this increase in forest area differs from other studies that found an overall forest loss in Spain and Portugal (Hansen et al. 2013). These previous studies examine general forest change rather than focusing only on broadleaf forests. Mixed or coniferous forest areas may have changed to broadleaf, appearing as habitat gain only in this study. Other possible causes of discrepancies might be the definition of forest area and the spatial and temporal resolution.
This increase in broadleaf forest area might be the result of recent agricultural land abandonment, afforestation activities, conservation policies, and the establishment of tree plantations. However, this net increase does not account for habitat quality. The gain of early successional or low heterogeneity forests may exceed the loss of mature and diverse forests, but not completely replace the ecosystem processes. In fact, many mature pure stands may be transformed into mono-species plantations (Teixido et al. 2010). These areas would be considered here as stable habitat patches besides the great loss of biodiversity and habitat quality. Furthermore, despite the total increase in broadleaf forest, the resistance of the surrounding landscape matrix was higher in 2018 than in 1990, reflecting the degradation of the interspersed matrix to land uses more impervious to focal species movements, such as urban areas or roads.
Time-varying connectivity: differences in connectivity between two snapshots
The results confirmed that connectivity changes over time as given by the varying value of overall connectivity (Fig. 5) and the different priority habitat patches between the two snapshots (Table S3 in supplementary material). These changes should be considered when planning optimal conservation measures to prolong the beneficial effects of the measures. In this study, landscape changes in habitat amount and distribution and in the surrounding matrix resulted in a large improvement in connectivity in the period analyzed from 1990 to 2018. There was a slightly larger increase in connectivity than in the total focal habitat area (Fig. S2). This might indicate that the habitat patches in 2018 were better connected because of the added habitat area per se (intrapatch connectivity) but also due to additional or improved connections between patches (interpatch connectivity). These improved connections might be the result of the gained habitat patches enhancing the connections with and between stable patches, or due to better characteristics of the intermediate matrix (even though there is an overall resistance increase, the matrix might have improved in-between the two connecting patches).
We found that landscape changes in most ecoregions had bigger impacts in connectivity for species with low dispersal distances (Fig. S2 in supplementary data): the gained or improved connections seem to be especially beneficial to short dispersers (Saura et al. 2011). Therefore, gained habitat patches may have been located close to the stable patches, acting as stepping stones and improving interpatch connectivity primarily for short dispersers. On the other hand, connectivity changes for vagile species were similar to the variation in habitat area. This might justify the focus of connectivity analyses in species with low dispersal abilities, which are more sensitive to landscape changes.
Spatio-temporal connectivity: connectivity between different time snapshots
Unlike static and time-varying approaches, spatio-temporal studies focus on connections between different snapshots. Following other approaches might lead to misjudging species connectivity to their future ranges in changing landscapes. The differing results obtained from static and spatio-temporal connectivity approaches suggest that static approaches fail to account for the connectivity between snapshots. The overall connectivity according to the spatio-temporal approach was in between that of the two static approaches, being in this case study generally smaller in 1990 and larger in 2018. Thus, the landscape changes (in the habitat patches distribution and area and the surrounding matrix) led to a large final static connectivity that did not especially favor the spatio-temporal connectivity between the two time snapshots (Fig. 2). The resulting final static overall connectivity tended therefore to overestimate spatio-temporal connectivity. However, when there was habitat loss (ecoregion 21, Southwest Iberian Mediterranean sclerophyllous and mixed forests) the use of static approaches underestimated the connectivity between snapshots. The same pattern of over and underestimation with net habitat gain and loss was found in other studies (Martensen et al. 2017; Huang et al. 2020).
The connectivity difference between the initial static and the spatio-temporal approaches decreased with the species dispersal distance (Figs. S1 and S5): species with low dispersal distances were more benefited from the spatio-temporal perspective than vagile species. This pattern was also found in other spatio-temporal connectivity studies (Martensen et al. 2017) and when comparing time-varying and static approaches (Fig. S2). This trend was particularly noticeable in Northwest Iberian montane forests (ecoregion 16), which even presented a loss in spatio-temporal connectivity for large dispersal distances (Fig. S5) despite the increase in habitat area (Table S2). This may be caused by the loss of links or stepping stones that connected distant nodes, which hindered the movement of long-distance dispersers. At the same time, the new habitat might have appeared close to other habitat patches already reachable for good dispersers and only enhancing the movement of species with low dispersal capacities.
When incorporating the temporal component to the probability of dispersal between patches, Martensen et al. 2017 considered the auxiliary links aside from the direct links here accounted. These auxiliary links do not provide spatio-temporal connectivity by themselves as they do not connect nodes active at t1 with nodes active at t2 (i.e., auxiliary links start at a gained node or finish at a lost node). However, they connect nodes that could coexist in time. The connection of these auxiliary links with other links could result in actual spatio-temporal connectivity. We did not consider this kind of link in our study, as their inclusion did not change the results and greatly increased computing times.
Patch prioritization
The results showed substantial differences in habitat prioritization between the two static approaches (Table S3 and Fig. S4). The key areas that maintain connectivity among contemporary patches largely varied from the initial to the final snapshots. Therefore, relying on traditional static connectivity models may lead to the prioritization of temporal suitable areas that would not persist (Wilson et al. 2009) under this condition for a long period. This finding further strengthens the idea of connectivity as a time-varying phenomenon (Saura et al. 2011; Beltrán et al. 2014; Bishop-Taylor et al. 2018b; Jennings et al. 2020).
The two dynamic approaches adopted here (i.e., time-varying and spatio-temporal) promoted connectivity between coexisting patches at several snapshots and from past to future patches. Although the two approaches accounted for landscape dynamics differently, they identified almost the same priority patches (Table S3, Fig. S4). That is to say, the patches with the highest long-term importance also contributed to a greater extent to species movements to their future ranges. The two dynamic approaches also captured neatly the priority areas from static landscape conditions at the two snapshots. Dynamic prioritization approaches are therefore useful to identify the key patches that promote connectivity at specific time snapshots, but they give extra consideration to patches critical at other time snapshots or with important spatio-temporal connections. However, only the spatio-temporal connectivity approach addresses all these issues by applying a unique model that remains useful despite changes in the landscape, while the time-varying prioritization approach requires running one model per time snapshot considered.
Further improvements
One of the main areas of improvement of the dynamic approaches is to add information about the landscape (the resistance of the matrix and the distribution, area, and quality of habitat patches) in-between 1990 and 2018. In particular, the spatio-temporal approach could benefit from more information about (a) the resistance of the matrix from 1990 to 2018 to obtain more accurate spatial probabilities of connectivity between habitat patches; and (b) the coexistence period between lost and gained patches to better capture their temporal probability of connectivity. Decreasing the time window between snapshots by including intermediate time snapshots (i.e., increasing the time resolution) could contribute to adding this information and therefore, further improve the functionality of the connectivity analyses. Including intermediate snapshots could also improve time-varying approaches by providing more detailed information about the perdurance of suitable habitats. The main limitation to including these intermediate snapshots is the long processing times of the least-cost path and connectivity metrics analyses. These analyses are particularly computationally intensive in the spatio-temporal scenario (with more nodes and connections). Code improvements to speed up the processing times, a smaller study area, or a coarser spatial resolution may allow including this intermediate information in the analyses. Less computationally intensive analyses would also make it possible to reduce the minimum path size (currently set at 50 ha) and to analyze movements among ecoregions.
Conclusions
This study emphasizes the importance of considering the dynamics of changing landscapes in connectivity studies. Traditional static approaches did not accurately assess the impacts of these changes. We have seen two different ways to incorporate these dynamics in connectivity studies: (a) by acknowledging that connectivity is a time-varying phenomenon and, therefore, providing long-term conservation measures; and (b) by enhancing species movements from their past to future ranges. We have shown that following different connectivity approaches resulted in very different overall connectivity values. Therefore, it is important to select the appropriate approach depending on the study objectives and the dynamic perspective considered. Otherwise, we might over or underestimate the connectivity levels in and between different time snapshots. On the other hand, habitat patches prioritization using the two dynamic perspectives showed very similar results. Both of them also identified most of the priority areas from static approaches at the two snapshots considered. Therefore, conservation strategies based on the two dynamic prioritization approaches may equally deal with the dynamics of landscapes and still enhance traditional static connectivity. That is to say, following either approach to incorporate the dynamics would foster endurable conservation measures that enhance connectivity between contemporary habitat patches, but also species range shifts in time. However, more research in this direction is needed to draw conclusions in other study areas, landscape changes, and time windows.
Data availability
The data that support the findings of this study are openly available at https://land.copernicus.eu/pan-european/corine-land-cover.
Code availability
The scripts used to generate the presented analyses are available in “figshare” at https://figshare.com/s/b9411312d964895deac3, https://doi.org/10.6084/m9.figshare.16799503.
References
Acevedo MA, Fletcher RJ, Tremblay RL, Meléndez-Ackerman EJ (2015) Spatial asymmetries in connectivity influence colonization−extinction dynamics. Oecologia 179:415–424
Adriaensen F, Chardon JP, De Blust G et al (2003) The application of “least-cost” modelling as a functional landscape model. Landsc Urban Plan 64:233–247
Albert CH, Rayfield B, Dumitru M, Gonzalez A (2017) Applying network theory to prioritize multispecies habitat networks that are robust to climate and land-use change. Conserv Biol 31:1383–1396
Ash E, Cushman SA, Macdonald DW et al (2020) How important are resistance, dispersal ability, population density and mortality in temporally dynamic simulations of population connectivity? A case study of tigers in Southeast Asia. Land 9:1–27
Banfield JE, Ciuti S, Nielsen CC, Boyce MS (2020) Cougar roadside habitat selection: incorporating topography and traffic. Glob Ecol Conserv 23:1–11
Beltrán BJ, Franklin J, Syphard AD et al (2014) Effects of climate change and urban development on the distribution and conservation of vegetation in a Mediterranean type ecosystem. Int J Geogr Inf Sci 28:1561–1589
Bishop-Taylor R, Tulbure MG, Broich M (2018a) Impact of hydroclimatic variability on regional-scale landscape connectivity across a dynamic dryland region. Ecol Indic 94:142–150
Bishop-Taylor R, Tulbure MG, Broich M (2018b) Evaluating static and dynamic landscape connectivity modelling using a 25-year remote sensing time series. Landsc Ecol 33:625–640
Bodin Ö, Norberg J (2007) A network approach for analyzing spatially structured populations in fragmented landscape. Landsc Ecol 22:31–44
Carroll C, Parks SA, Dobrowski SZ, Roberts DR (2018) Climatic, topographic, and anthropogenic factors determine connectivity between current and future climate analogs in North America. Glob Chang Biol 24:5318–5331
Chen IC, Hill JK, Ohlemüller R et al (2011) Rapid range shifts of species associated with high levels of climate warming. Science (80-) 333:1024–1026
Cisneros-Araujo P, Goicolea T, Mateo-Sánchez MC et al (2021) The role of remote sensing data in habitat suitability and connectivity modeling: Insights from the cantabrian brown bear. Remote Sens. https://doi.org/10.3390/rs13061138
Collinge SK (1996) Ecological consequences of habitat fragmentation: implications for landscape architecture and planning. Landsc Ecol 36:59–77
Conlisk E, Haeuser E, Flint A et al (2021) Pairing functional connectivity with population dynamics to prioritize corridors for Southern California spotted owls. Divers Distrib. https://doi.org/10.1111/ddi.13235
Correa Ayram CA, Mendoza ME, Etter A, Pérez Salicrup DR (2015) Habitat connectivity in biodiversity conservation: a review of recent studies and applications. Prog Phys Geogr 40:1–32
Costanza JK, Watling J, Sutherland R et al (2020) Preserving connectivity under climate and land-use change: No one-size-fits-all approach for focal species in similar habitats. Biol Conserv. https://doi.org/10.1016/j.biocon.2020.108678
Cushman SA (2015) Pushing the envelope in genetic analysis of species invasion. Mol Ecol 24:259–262
Davidson SC, Bohrer G, Gurarie E et al (2020) Ecological insights from three decades of animal movement tracking across a changing Arctic. Science 370:712–715
de la Fuente B, Mateo-Sánchez MC, Rodríguez G et al (2018) Natura 2000 sites, public forests and riparian corridors: the connectivity backbone of forest green infrastructure. Land Use Policy 75:429–441
Dondina O, Orioli V, Colli L et al (2017) Ecological network design from occurrence data by simulating species perception of the landscape. Landsc Ecol 33:275–287
Drake J, Lambin X, Sutherland C (2021) The value of considering demographic contributions to connectivity: a review. Ecography (cop). https://doi.org/10.1111/ecog.05552
Elsen PR, Monahan WB, Dougherty ER, Merenlender AM (2020) Keeping pace with climate change in global terrestrial protected areas. Sci Adv. https://doi.org/10.1126/sciadv.aay0814
Gastón A, Blázquez-Cabrera S, Ciudad C et al (2019) The role of forest canopy cover in habitat selection: insights from the Iberian lynx. Eur J Wildl Res 65:1–10
Gray M, Micheli E, Comendant T, Merenlender A (2020) Climate-wise habitat connectivity takes sustained stakeholder engagement. Land 9:1–21
Gurrutxaga M, Saura S (2013) Prioritizing highway defragmentation locations for restoring landscape connectivity. Environ Conserv 41:157–164
Gurrutxaga M, Rubio L, Saura S (2011) Landscape and Urban Planning Key connectors in protected forest area networks and the impact of highways: a transnational case study from the Cantabrian Range to the Western Alps ( SW Europe ). Landsc Urban Plan 101:310–320
Hansen MC, Potapov PV, Moore R et al (2013) High-Resolution Global Maps of 21st-Century Forest Cover Change. Science (80-) 342:850–853
Honnay O, Verheyen K, Butaye J et al (2002) Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecol Lett 5:525–530
Huang JL, Andrello M, Martensen AC et al (2020) Importance of spatio–temporal connectivity to maintain species experiencing range shifts. Ecography (cop) 43:591–603
Jennings MK, Haeuser E, Foote D et al (2020) Planning for dynamic connectivity: operationalizing robust decision-making and prioritization across landscapes experiencing climate and land-use change. Land 9:341
Keeley ATH, Beier P, Gagnon JW (2016) Estimating landscape resistance from habitat suitability: effects of data source and nonlinearities. Landsc Ecol 31:2151–2162
Keeley ATH, Ackerly DD, Basson G, et al (2018a) Landscape connectivity: why , how, and what next
Keeley ATH, Ackerly DD, Cameron DR et al (2018b) New concepts, models, and assessments of climate-wise connectivity New concepts, models, and assessments of climate-wise connectivity. Environ Res Lett 13:1–18
Keeley ATH, Beier P, Jenness JS (2021) Connectivity metrics for conservation planning and monitoring. Biol Conserv. https://doi.org/10.1016/j.biocon.2021.109008
Lawler JJ, Ruesch AS, Olden JD, Mcrae BH (2013) Projected climate-driven faunal movement routes. Ecol Lett 16:1014–1022
Littlefield CE, McRae BH, Michalak JL et al (2017) Connecting today’s climates to future climate analogs to facilitate movement of species under climate change. Conserv Biol 31:1397–1408
Loarie SR, Duffy PB, Hamilton H et al (2009) The velocity of climate change. Nature 462:1052–1055
Martensen AC, Saura S, Fortin MJ (2017) Spatio-temporal connectivity: assessing the amount of reachable habitat in dynamic landscapes. Methods Ecol Evol 8:1253–1264
Mateo Sánchez MC, Cushman SA, Saura S (2014) Scale dependence in habitat selection: the case of the endangered brown bear (Ursus arctos) in the Cantabrian Range (NW Spain). Int J Geogr Inf Sci 28:1531–1546
Mateo-Sánchez MC, Balkenhol N, Cushman S et al (2015) A comparative framework to infer landscape effects on population genetic structure: are habitat suitability models effective in explaining gene flow? Landsc Ecol 30:1405–1420
Mora C, Frazier AG, Longman RJ et al (2013) The projected timing of climate departure from recent variability. Nature 502:183–187
Nuñez TA, Lawler JJ, McRae BH et al (2013) Connectivity planning to address climate change. Conserv Biol 27:407–416
Olson DM, Dinerstein E, Wikramanayake ED et al (2001) Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51:933–938
Parks SA, Carroll C, Dobrowski SZ, Allred BW (2020) Human land uses reduce climate connectivity across North America. Glob Chang Biol 26:2944–2955
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Pascual-Hortal L, Saura S (2006) Comparison and development of new graph-based landscape connectivity indices: towards the priorization of habitat patches and corridors for conservation. Landsc Ecol 21:959–967
Phillips SJ, Williams P, Midgley G, Archer A (2008) Optimizing dispersal corridors for the cape proteaceae using network flow. Ecol Appl 18:1200–1211
Poli C, Hightower J, Fletcher RJ (2019) Validating network connectivity with observed movement in experimental landscapes undergoing habitat destruction. J Appl Ecol. https://doi.org/10.1111/1365-2664.13624
Román-Palacios C, Wiens JJ (2020) Recent responses to climate change reveal the drivers of species extinction and survival. PNAS. https://doi.org/10.1073/pnas.1913007117
Root TL, Price JT, Hall KR et al (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Ruiz-González A, Gurrutxaga M, Cushman SA et al (2014) Landscape genetics for the empirical assessment of resistance surfaces: The European pine marten (Martes martes) as a target-species of a regional ecological network. PLoS ONE. https://doi.org/10.1371/journal.pone.0110552
Santini L, Di Marco M, Visconti P et al (2013) Ecological correlates of dispersal distance in terrestrial mammals. Hystrix, Ital J Mammal Online. https://doi.org/10.4404/hystrix-24.2-8746
Santini L, Saura S, Rondinini C (2016) Connectivity of the global network of protected areas. Divers Distrib 22:199–211
Saura S (2007) A new habitat availability index to integrate connectivity in landscape conservation planning: comparison with existing indices and application to a case study. Landsc Urban Plan 83:91–103
Saura S, Pascual-Hortal L (2007) A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landsc Urban Plan 83:91–103
Saura S, Rubio L (2010) A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography (cop) 33:523–537
Saura S, Torné J (2009) Conefor Sensinode 2.2: a software package for quantifying the importance of habitat patches for landscape connectivity. Environ Model Softw 24:135–139
Saura S, Estreguil C, Mouton C, Rodríguez-Freire M (2011) Network analysis to assess landscape connectivity trends: application to European forests (1990–2000). Ecol Indic 11:407–416
Saura S, Bastin L, Battistella L et al (2017) Protected areas in the world’s ecoregions: how well connected are they ? Ecol Indic 76:144–158
Saura S, Bertzky B, Bastin L et al (2018) Protected area connectivity: shortfalls in global targets and country-level priorities. Biol Conserv 219:53–67
Song X, Hansen MC, Stehman SV et al (2018) Global land change 1982–2016. Nature 560:639–643
Spear SF, Balkenhol N, Fortin MJ et al (2010) Use of resistance surfaces for landscape genetic studies: considerations for parameterization and analysis. Mol Ecol 19:3576–3591
Sutherland GD, Harestad AS, Price K, Lertzman KP (2000) Scaling of natal dispersal distances in terrestrial birds and mammals. Ecol Soc. https://doi.org/10.5751/es-00184-040116
Taylor RG, Scanlon B, Döll P et al (2013) Groundwater and climate change: recent advances and a look forward. Nat Clim Chang 3:322–329
Teixido AL, Quintanilla LG, Carreño F, Gutiérrez D (2010) Impacts of changes in land use and fragmentation patterns on Atlantic coastal forests in northern Spain. J Environ Manage 91:879–886
Tischendorf L, Fahrig L (2000) On the usage and measurement of landscape connectivity. Oikos 1:7–19
Urban DL, Keitt T (2001) Landscape connectivity: a Graph-theoretic perspective. Ecology 82:1205–1218
Urban DL, Minor ES, Treml EA, Robert S (2009) Graph models of habitat mosaics. Ecol Lett 12:260–273
Williams P, Hannah L, Andelman S et al (2005) Planning for climate change: identifying minimum-dispersal corridors for the cape proteaceae. Conserv Biol 19:1063–1074
Wilson KA, Cabeza M, Klein CJ (2009) Fundamental concepts of spatial conservation prioritization. In: Spatial Conservation Prioritization: Quantitative Methods and Computational Tools. pp 16–27
Zeller KA, McGarigal K, Whiteley AR (2012) Estimating landscape resistance to movement: a review. Landsc Ecol 27:777–797
Zeller KA, Lewsion R, Fletcher RJ et al (2020) Understanding the importance of dynamic landscape connectivity. Land 9:1–15
Zhao H, Liu D, Li F et al (2021) Incorporating spatio-temporal connectivity for prioritized conservation of individual habitat patches in a dynamic landscape. Ecol Indic 124:107414
Acknowledgements
We gratefully acknowledge the Universidad Politécnica de Madrid for the funding and for providing computing resources on Magerit Supercomputer.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by funding from the Universidad Politécnica de Madrid.
Author information
Authors and Affiliations
Contributions
All authors conceived the ideas and designed the methodology; TG analyzed the data and ran the connectivity models with the help of MCM. TG led the writing of the manuscript and MCM reviewed it thoroughly. Both authors critically contributed to the drafts and gave their final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable. However, the authors comply with the IUCN Policy Statement on Research Involving Species at Risk of Extinction.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goicolea, T., Mateo-Sánchez, M.C. Static vs dynamic connectivity: how landscape changes affect connectivity predictions in the Iberian Peninsula. Landsc Ecol 37, 1855–1870 (2022). https://doi.org/10.1007/s10980-022-01445-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01445-5