Abstract
Context
Habitat loss and fragmentation can interact with other threats, including altered fire regimes, and responses to these effects can be mediated by functional traits.
Objectives
To determine how richness and abundance of reptile trait groups respond to habitat fragmentation, patch isolation and fire.
Methods
We surveyed reptiles in 30 sites over 3 years. Sites in remnant patches in farmland were adjacent to a conservation park with either recently burnt or long-unburnt habitat. The remnant patches were stratified by distance from the reserve. Sites were spatially paired, and we experimentally burnt one of each pair in farmland. Trait groups included size, reproduction, habitat position, diet, and activity period.
Results
None of the trait groups benefited from experimental burns, while the burns reduced abundance of viviparous, small, and above-ground species. Species richness was lower in isolated sites than in sites close to the conservation park, while generalist trait groups appeared unaffected by patch isolation. Large-sized reptiles had higher abundance in remnants. There was not more rapid colonisation of burnt sites near recently burnt conservation park. Instead, low initial abundance may have been caused by fire in combination with drought, with high rainfall during the study allowing recovery and spill-over into adjacent remnants.
Conclusions
Landscape structure appears to interact with natural fires, restoration burns and longer-term climatic trends to influence the abundance and distribution of reptiles. Traits mediate responses, enabling us to formulate a set of testable mechanistic hypotheses, which illustrates a pathway to generalisation and prediction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation are major drivers of biodiversity loss, and rising demand for resources along with growing human populations will see habitat loss increase in many parts of the world (Williams et al. 2021a). The detrimental impacts of habitat loss and fragmentation are partly due to loss of habitat area but also ecosystem decay (Haddad et al. 2015; Chase et al. 2020). As remnant vegetation becomes smaller and more isolated, the quality of habitat can degrade through edge effects, including altered microclimates and spill-over of farm-adapted species (Driscoll et al. 2013), and disturbances, such as livestock grazing and inappropriate fire regimes (Ewers and Didham 2006). When acting concurrently, these additional threatening processes can modify the effects of habitat loss and fragmentation (Geary et al. 2019).
Altered fire regimes, for example, can act with habitat loss and fragmentation to modify the impacts that either would have alone (Driscoll et al. 2021). Their combined effects can be detrimental, with fire causing local extinctions and fragmentation preventing populations from re-establishing (Alstad and Damschen 2016), or their combined effects can exceed an optimal intermediate level of disturbance (Scroggie et al. 2019). Conversely, fire suppression can drive fragmentation and addition of fire can restore connectivity for species that use more open habitats (Neuwald and Templeton 2013). However, successful restoration of connectivity with fire can depend on proximity to source populations, with patches close to sources receiving more immigrants than isolated sites (MacArthur and Wilson 1967; Hanski 1998; Leibold et al. 2004). Colonisation of unoccupied patches can also depend on the similarity of successional stage between patches and potential sources, because species often have strong affiliations with particular successional stages of vegetation recovery (Smith et al. 2013). However, knowledge of such spill-over effects is limited (Matthews 2021).
An important approach to generate transferable knowledge has been to use traits to identify groups of species that are vulnerable to the effects of habitat disturbance (Keinath et al. 2017). For reptiles—a group threatened by habitat loss, fragmentation and modification (Keinath et al. 2017; Doherty et al. 2020), particularly agriculture (Chapple et al. 2021; Wong et al. 2021)—a range of traits have proven useful in discriminating responses, including body size, reproduction, diet, habitat position and activity period (Watling and Donnelly 2007; Santos and Cheylan 2013; Carvajal-Cogollo and Urbina-Cardona 2015; Bohm et al. 2016; Neilly et al. 2018; Val et al. 2019; Chergui et al. 2020; Williams et al. 2021b). While substantial research effort has been directed towards understanding the role of traits in explaining reptile responses to disturbance, consistent trends have not yet emerged. More cases describing the circumstances in which particular traits influence responses to fire, habitat loss and fragmentation are needed to help develop contingent theory (Driscoll and Lindenmayer 2012).
Parts of the semi-arid mallee woodlands of southern Australia have been extensively cleared for agriculture, leaving linear remnants and small patches that experience fire suppression (Driscoll and Henderson 2008; Williams et al. 2012). Adjacent parts consist of large tracts of mallee in nature reserves that have natural fire return intervals of decades to centuries, depending on rainfall (Gibson et al. 2015). The reptile fauna is rich, and many species have strong responses to both fire and habitat fragmentation (Driscoll 2004; Nimmo et al. 2012; Smith et al. 2013). With this combination of intact woodlands adjacent to fire-suppressed remnant patches, mallee landscapes are ideal for examining how traits of reptiles influence their response to the combination of habitat fragmentation and experimental fire added as a potential restoration tool.
Our specific aims were to:
-
(1)
Investigate how the addition of fire to remnant vegetation affects the abundance and richness of different reptile trait groups.
-
(2)
Quantify the effects of patch isolation on richness and abundance of reptile trait groups.
-
(3)
Determine the overall effects of habitat conversion to farmland on reptiles in remnant vegetation by comparing reserves with remnants.
-
(4)
Determine how the effects of fire in remnants are moderated by potential source populations in adjacent reserves, using reserves at different stages of post-fire succession.
Given that reptile trait groups show diverse responses to fire, and research has not always included traits we have available, it is difficult to make predictions about responses to the addition of fire to remnant vegetation (aim 1). However, with predicted detrimental impacts of an agricultural matrix on remnants (Driscoll et al. 2013), small patch size (Doherty et al. 2020), and the likely limited dispersal of reptiles in our study (Williams et al. 2012; Driscoll et al. 2012b), we expected that richness and abundance of most trait groups would be lowest in the most isolated remnants (aim 2) and lower in the remnant vegetation compared with the conservation park (aim 3). Possible exceptions would be traits that confer some advantage in disturbed landscapes, such as generalist habits (Watling and Donnelly 2007; Bohm et al. 2016). We expected that experimentally burnt patches close to the recently burnt conservation park would gather colonists faster than recently burnt patches adjacent to long unburnt mallee (aim 4) (Gentry and Vierling 2007).
The impacts of habitat fragmentation and loss are global and worsening as they interact with other threats including climate change (Segan et al. 2016), invasive or irruptive species (Geary et al. 2019) and altered fire regimes (Driscoll et al. 2021). Understanding how these interactions affect biodiversity depends on knowledge of landscape processes, including the effects of patch isolation, patch condition and sources of colonists. Our study, a combination of natural and manipulative experiment in a large-scale field setting, aims to improve knowledge of the ecology of adding fire to fragmented landscapes.
Methods
Study area
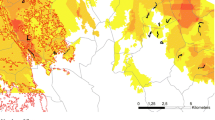
Our study was conducted in Pinkawillinie Conservation Park (33° 05′ 41.05″ S, 135° 59′ 57.75″ E) and remnant native vegetation on linear sand dunes in the adjacent farmland in the northern Eyre Peninsula, South Australia (Fig. 1). The region has a semi-arid climate, with a mean annual rainfall of 265 mm. The low topographic relief comprises shallow soils that are mainly siliceous and calcareous sands (Blackburn and Wright 1989). The vegetation consists of mallee woodlands dominated by multi-stemmed eucalypts (predominantly Eucalyptus costata and E. socialis), often with shrubs (Melaleuca uncinata) and spinifex (Triodia irritans) (Robinson and Heard 1985). Remnants in farmland are subject to disturbances associated with cropping (weeds, pesticides, fertiliser) and livestock grazing, reducing shrub cover and plant species richness (Schutz and Driscoll 2008; Driscoll et al. 2012b), but there are no fires recorded in recent history at these sites. A bushfire burnt the northern section of the park in 2005, 5 years before our surveys began (Fig. 1).
Study area showing A the study region on the semi-arid Eyre Peninsula in southern Australia, B the layout of sites in Pinkawillinie Conservation Park and C the layout of transects in one of the northern blocks. The light grey shading within B indicates the recently burnt woodland (i.e. burnt in 2005); the dark-shaded area within the reserve border is long unburnt woodland. Reptiles were surveyed along a total of 30 transect sites that were paired and established in dunes and swales in the reserve and in remnant dunes in the cropping matrix (C). Surveys took place in 2010 before experimental burns and in the subsequent 2 years
Survey design
Our survey design consisted of three blocks in the south and three in the north of the study area (Fig. 1). Each southern block included six sites; a dune and swale (inter-dune) site within the conservation park in an area that had no fire records within the past 38 years, two dunes in farmland close to the conservation park (0.05–2.7 km), and two dunes far from the conservation park in farmland (5.5–8.8 km, details in Online Resource 1 in supporting information). Each northern block included a dune and swale site in the conservation park in an area burnt 5 years previously, and two dune sites close to the conservation park in farmland (0.18–1.6 km). All dune-top remnants embedded in farmland were long-unburnt. Patch area did not differ among treatments (ANOVA, with patch area of sites embedded in farmland as response, and a six-level factor delimiting each treatment; F5,12 = 1.26, P = 0.34).
One of the two dunes at each level of isolation (near, far) in each block was selected by landholders for an experimental burn, with one exception where the landholder did not give permission to burn a far site (Fig. 1). After surveys in early 2010, northern sites were burnt on 26th March 2010 and southern sites the next day. Fires were set under mild conditions with winds < 10 km/h, humidity 40–80%, and with low fuel loads (~ 2 tonnes/ha). The fires rarely entered the tree canopy and often did not carry unassisted across the ground due to an absence of fuel (Fig. 2). The fires were therefore atypical of natural fires in mallee ecosystems which tend to be large and consume the entire canopy. Dunes within conservation parks were not burnt due to risks of fire escaping into the park.
We surveyed each site for two 14-day trapping sessions (i.e., 28 nights total) in each of three consecutive summers (2010, 2011 and 2012). We sampled dunes in the conservation park and adjacent farmland, but swales were only in the reserve. All swale habitat was cleared for cropping in farmland and few reptiles use cleared land (Driscoll 2004; Schutz and Driscoll 2008). We sampled swales to understand the pool of nearby species that could potentially colonise dunes in farmland.
At each of the 30 sites, we surveyed reptiles using ten 20 L pitfall traps spaced 25 m apart, each with a 30 cm high × 10 m long, plastic drift fence, placed at alternate right angles. We also placed a 15 cm long half PVC pipe in the bottom of each bucket with a 15 × 20 cm wood block to act as a shelter from heat and that could float in case of rain. We uniquely marked trapped animals using implanted elastomer so that we could omit recaptures from the counts. We released marked animals at the point of capture the following day. Trapped individuals were identified to species, except for Pogona species, which have unresolved taxonomy in our study area (Driscoll and Henderson 2008).
Traits
We used published literature and personal observations to assign traits relevant to reptile fire responses (Smith et al. 2013; Meiri 2018) to each species. These traits included body size (small—< 66 mm snout-vent length; medium—66–140 mm; large—> 190 mm), reproduction (oviparous, viviparous), habitat-position (above-ground—semi-arboreal or living on the ground or in leaf litter; below-ground—species that use burrows or are fossorial), diet (carnivorous; insectivorous; omnivorous), and activity period (diurnal; nocturnal (includes crepuscular); both day and night active) (Online Resource 2). We limited traits to those for which we had data for all species. We calculated species richness and total abundance for each trait category to use as response variables.
Analysis
We combined data from the two trapping sessions within each year. We used the secondary candidate set strategy to identify the most plausible models (Morin et al. 2020), based on generalised linear and generalised linear mixed models (Bolker et al. 2009). To identify random effects models to carry through to the second phase of analysis, we fitted all additive and nested combinations of site, pair and block to each response variable and retained models within six Akaike's Information Criterion for small samples (AICc) of the best model (Burnham and Anderson 2002).
Fixed effects in the models included combinations of variables that delimited our study design (Fig. 1) plus appropriate two and three-way interactions. We included topographic position (dune vs swale) in our analysis to account for that source of variation, rather than to explore its specific effects. Main effects were: Region (South—where the conservation park was long unburnt, North—conservation park recently burnt); Tenure (Park, Farm); Topographic Position (Dune, Swale); Isolation (Close, Far); Burn (Unburnt, Burnt); Year1 (2010 before burn treatments, 2011–2012 after burns implemented), and; Year (2010, 2011, 2012, a variable fitted as a more detailed alternative to Year1). Interactions included: Tenure:Region; Region:Burn; Year1:Burn; Year1:Far; Year1:Region; Year1:Tenure; Year1:Region:Burn; Year1:Region:Tenure. We included models with all possible combinations of the interactions. Models including Year1 were repeated but with Year instead. Although we intended to examine Year:Far:Burn interactions, we did not because one of three planned far-burnt sites was not burnt. We did not use Year1 and Year in the same models (variables we refer to as ‘time’) and when both Year1 and Year (or interactions) were among the best models, we present results for Year to avoid repetition. We retained models with delta AICc ≤ 6 for the second phase of analysis.
In the second phase of analysis, we explored all possible combinations of the best random effects models and the best fixed effects models in mixed-effects models. We ranked these using AICc, then averaged estimates over models with delta AICc ≤ 2. We plotted results for variables with P ≤ 0.05.
To avoid over-fitting, we limited the models fitted to each response to those where the number of parameters in the model was less than one third of the number of non-zero response values. This resulted in the 30 most complex models being dropped for viviparous reptiles, and the three most complex models being dropped for carnivorous reptiles. By only interpreting results with P < 0.05, the set of parameters excluded from interpretation included those that could be classified as uninformative (Leroux 2019). We converted responses with five or fewer values > 2 or with < 10 unique values to presence/absence data and analysed assuming a binomial distribution of errors with a logit link function (only carnivorous reptile abundance met this criteria). We analysed all other responses assuming Poisson distribution of errors with a log-link function. We assessed over-dispersion using a Pearson Chi-squared test of Pearson residuals divided by residual degrees of freedom (Maindonald and Braun 2010). No models had significant evidence of over-dispersion. We conducted analyses in R (R Core Team 2021) using libraries lme4 (glmer) (Bates et al. 2015); bbmle (AICctab) (Bolker and R Development Core Team 2020), rsq (rsq) (Zhang 2021), effects (effect), car (Anova) (Fox and Weisberg 2019), and MuMin (model.avg, AICc) (Barton 2020).
The package MuMIn has a predict function that does not average over levels of categorical variables, and therefore requires arbitrary choices about which level of categorical variables to include in predictions for plotting results. Instead, we use the effects package to obtain predicted values and confidence limits from the best model in which a particular variable occurred. The variables plotted were nevertheless limited to those with P < 0.05 based on model averaging. The estimates were in the same direction and within 10% of one another (mean ratio of model-averaged vs best model estimates = 0.99, SD = 0.07), with five exceptions (Online Resource 3).
Results
Overall, we recorded 2200 reptiles from 42 species in seven families: geckoes (822, 3 species); skinks (637 individuals, 21 species); dragons (512 individuals, 6 species); blind snakes (111 individuals, 2 species); elapid snakes (71 individuals, 5 species); goannas (33 individuals, 1 species) and legless lizards (14 individuals, 4 species). In 2010, 2011 and 2012, we recorded 685, 707 and 808 reptiles respectively.
Aim 1: fire-time interactions
Four reptile response variables had fire-time interactions, representing effects of our experimental burns in remnant patches (see Online Resource 4 for ranked models, and Online Resource 5 for model averaged estimates). Abundance of viviparous species declined after fire (Fig. 3A). The abundance of small reptiles and those that live on or above the ground showed a substantial increase over time in unburnt sites, but this trend was not observed at burnt sites (Fig. 3B, C). There was weak evidence that abundance was beginning to recover by the 3rd year (Fig. 3B, C). Nocturnal reptiles had very low abundance on sites planned for burning in 2010 in the northern blocks, and abundance increased on those sites after they were burnt (Fig. 3D).
Predicted mean abundance of trait groups for which an interaction of burn and year was in the best model. Headers for each panel indicate the dependent variable and P values based on model averaged estimates. Error bars indicate 95% confidence intervals. U unburnt, B burnt, S South, N North, 11–12 2011–2012. Experimental burns took place after the 2010 surveys
Aim 2: patch isolation
On far sites, there were fewer species overall and fewer species of three trait groups (small, above-ground and diurnal; Fig. 4B, C, G, I). Increasing trends in abundance of all reptiles (Fig. 4A) and small species (Fig. 4D) in close sites contrasted with abundance in far sites which did not show increases. Four other trait groups showed patterns of decline in far sites over time (medium, below-ground, insectivores, day and night active, Fig. 4E, F, H, J), but with high initial abundance in far sites and weak or fluctuating changes over time in close sites.
Aim 3: habitat conversion; conservation park vs farms
Abundance of all reptiles (Fig. 5A), oviparous and on/above-ground reptiles (Fig. 5B, F) increased over time in the conservation park but showed weaker responses in farm remnants. Abundance of viviparous species was higher in the park than in farmland in 2011 due to a marked drop in numbers on farmland and high average numbers in the park (Fig. 5C). There were fewer species of medium-sized reptiles (Fig. 5D) in farm remnants. Large species had higher abundance in farm remnants compared with the conservation park, with increasing numbers over time (Fig. 5E). Omnivorous reptiles had lower abundance in farms than parks, but only in the northern region, with the opposite pattern in the south (Fig. 5G).
Predicted mean abundance and mean species richness of trait groups for which tenure (conservation park vs farm) was in the best model, or in interaction with year or region (north, south). Headers for each panel indicate the dependent variable and P values. Error bars indicate 95% confidence intervals. S South, N North
Aim 4: proximity to recently burnt or long unburnt conservation park
Abundance in the northern region tended to be lower in the 1st year than in the south (except for diurnal species), then increased over time, in some cases becoming more abundant than in the south. This general trend was seen for total number of reptiles and oviparous, large, below, on or above, omnivorous and diurnal reptiles (Fig. 6A, B, E–I). Similar trends were shown by medium and day and night active species, but the latter had highest abundance in 2011, and both showed declines in the south (Fig. 6D, J). Small reptiles were consistently less abundant in the north (Fig. 6C).
Main effects of burn, time, and topographic position
We did not expect to see main effects of burn (without interaction with time) in our results because the burns took place after the 1st year of data were collected. These main effects indicate our burnt sites were different throughout the study, including before the burn treatment was implemented. Burnt sites had fewer species of small, above-ground, insectivorous and diurnal species. Burn sites also had lower abundance of large reptiles, and in the north burnt sites, lower abundance of all reptiles, oviparous, medium, below ground, and insectivorous species (Online Resource 6).
Over the 3 years of the study, there was an increase in reptile species richness, richness of nine trait groups (oviparous, small, large, below-ground, above-ground, insectivorous, carnivorous, diurnal, and nocturnal) and occurrence of carnivores (Online Resource 7). Four trait groups responded to topographic position, with dunes supporting higher abundance of medium-sized, below-ground, and nocturnal reptiles but fewer large-bodied reptiles than in swales (Online Resource 8).
Discussion
Our study confirms the strong negative effects that habitat loss, fragmentation and patch isolation have on reptiles (Keinath et al. 2017). By examining traits, we can provide some guidance about reptiles that may be most vulnerable or resilient to habitat modification, as well as insight into the possible mechanisms for these responses. Experimental fires in general were not beneficial within the timeframe of our study, and there were some detrimental effects. However, in comparing the southern region that had long-unburnt habitat with the northern region that had recently burnt habitat, we reveal patterns that suggest fire in reserves could have landscape-scale effects into the nearby farmland remnants.
Fire
Fire in remnant vegetation was associated with declines in viviparous species, and reductions in small and above-ground species at a time when these groups were increasing on unburnt sites. That species living on or above ground, rather than those living underground, were sensitive to fire is expected: species living in the strata of the environment that are disturbed should be most sensitive to the disturbance (Driscoll and Weir 2005; Neilly et al. 2018). Given the canopy did not burn in our experimental fires, the observed impact on above-ground species may be smaller than would occur in a wildfire. Body size rarely affects reptile responses to disturbance (Wang et al. 2009; Martin and Murray 2011; Doherty et al. 2020; Hu et al. 2020). However, small species can be vulnerable to extinction if they experience rapid drops in population growth rates (Williams et al. 2021b), potentially explaining reduced abundance after fire in small but not larger species. Finally, although oviparous reptiles are expected to be more vulnerable to disturbance because incubation temperatures cannot be controlled and nest sites can be lost (Doherty et al. 2020), it was viviparous species that declined after our experimental burns. We suggest that some of the oviparous species had eggs below ground at the time of our fires (e.g. Smith et al. 2012), reducing the impact of fire on reproduction. Further, 65% of oviparous individuals were burrowers or fossorial whereas 47% of viviparous individuals were, meaning more viviparous individuals may have been directly exposed to fire.
Low abundance of nocturnal reptiles before experimental burning suggests that factors other than fire dominated abundance of these groups, driving low numbers in the north in the 1st year. However, we expected that recovery after fire in the north would be aided by proximity to recently burnt habitat. If this hypothesis was correct, we would expect species that increased in abundance on burnt remnants in the north to also have low abundance on unburnt remnants, and high source populations in the reserve in the north. However, there were no data for individual species that were consistent with this expectation (data not shown), so this colonisation hypothesis is unlikely. We offer a tentative explanation in the final section of this discussion.
Most trait groups in our study did not respond to experimental burning. These muted fire responses could be caused by variation among the species within trait groups (Santos et al. 2014). Of the 42 species we trapped, 16 species have a published fire response (Driscoll and Henderson 2008; Nimmo et al. 2012; Driscoll et al. 2012a; Smith et al. 2013). Most of our trait groups included both early and late successional species (e.g. insectivores had five early and eight late successional species; diurnal had five early and four late, above-ground had two early and five late). By taking a focus on single trait groups, fire responses at the trait level could be obscured because species with the same traits nevertheless have different fire responses. In future, considering interactions among traits or trait combinations could be a more insightful approach to predicting fire responses. However, large datasets with many species are required, making that a challenging approach (Driscoll et al. 2020).
Sites that were planned for burning had differences from unburnt patches before we burnt them. This was most likely related to initial site selection where farmers gave permission to burn one of two remnants in a non-random way, often selecting the most degraded of two remnants for burning. Our results for burning by year interactions therefore have the caveat that they represent responses of reptiles on degraded remnants. Further work is needed to understand if reptiles show a different response if remnants in good condition are burnt.
Patch isolation
The most isolated sites had lower species richness, lower richness for three trait groups (diurnal, small, above-ground) and lower abundance of small species. Reduced species richness in more isolated sites is predicted by island biogeography theory (MacArthur and Wilson 1967), metacommunity theories that invoke dispersal limitation (Leibold et al. 2004), and metapopulation theory applied to multiple species (Driscoll 2008). Small populations in remnant patches have a substantial risk of extinction and where dispersal is limited so that recolonization is prevented, species can be permanently lost from the landscape (Hanski 1998). In our study system, this process was previously illustrated by the knob tailed gecko Nephrurus stellatus, which had high occurrence rates in patches within 300 m of occupied sites but very low occurrence in patches further than 700 m from occupied sites (Driscoll et al. 2012b). Further, distance-limited spill-over (Leibold et al. 2004; Lucey et al. 2014) from the conservation park could explain increasing abundance of small reptiles in close sites, but not far sites.
For the trait groups without significant effects of patch isolation on richness, we further examined average species richness in close and far sites. Almost all reptile trait groups had substantially lower average richness in far sites compared with close sites (Online Resource 9a). We suggest the lack of a significant effect in these trait groups could be due to limited statistical power. However, species that were omnivorous, medium in size or active both day and night had substantially smaller mean differences than other groups, so these groups may be more robust to patch isolation. Omnivores and species that can be active day or night are more flexible in their life strategies than other trait groups (Santos et al. 2014), potentially providing advantages in more isolated sites. Generalists typically do better in disturbed sites and have lower risk of extinction than specialist species (Bohm et al. 2016; Palmeirim et al. 2017a). One potential explanation for minimal decline in richness of medium-sized reptiles in isolated sites is predator release. Lion et al (2016) suggested the absence of key avian predators from small Atlantic Forest remnants may have contributed to higher reptile richness. In our study, predator release potentially counterbalances the otherwise negative effects of isolation.
Predator release could also explain why abundance of medium, below-ground, insectivorous and reptiles active in day and night had high abundance in far sites in the 1st year, then declined. The importance of predation in community organisation is expected to increase as resources increase, while the role of competition should decrease (Bohannan and Lenski 2000; Letnic et al. 2013). Rainfall, and thus resources, were very low prior to our study during the millennial drought (average rainfall 2002–2008 210 mm (range 174–251 mm), Buckleboo station, Australian Bureau of Meteorology). However, in 2009, 2010 and 2011, rainfall at Buckleboo was 48%, 90% and 71% higher respectively than the drought average. With high rainfall after a period of drought, predation pressure may have increased in isolated sites over time. This hypothesis raises the suggestion that predators expand outwards from the reserve and close sites in high rainfall years, altering community composition in isolated sites.
Although we could not test for an interaction of burn with isolation, average numbers (Online Resource 9b) suggest that the burn in far sites did not cause substantial declines in medium, below-ground and day–night active reptiles. However, the low numbers of insectivores in far sites in 2011 and 2012 could be related to an interaction of burn and isolation, an alternative to the predator release hypothesis. In insectivores, the change over time was small on close sites, regardless of burn treatment, but on far sites, declines were twice as large on burnt (48% decline) compared with unburnt sites (25% decline, Online Resource 9b). Possibly insects were less abundant immediately after the fires in far sites, although, 4 years after fire, insect numbers can be as high or higher than in unburnt, unfragmented mallee (Teasdale et al. 2013).
Habitat conversion
Our comparison of conservation parks and farms highlight the overall effect of landscape conversion from natural vegetation to agriculture. Remnants in farmland experience a range of different conditions compared with conservation parks. Hansen et al. (2019) reported elevated attacks on reptile models by mammals and birds along remnant edges in Australian woodlands, while other studies have found higher predation risk in open areas (Bateman et al. 2017). Mallee remnants in farmland have more weeds (Davies et al. 2013), less native vegetation cover (Driscoll 2004; Schutz and Driscoll 2008), foundation plant species are less common (Bell et al. 2021) and are likely to experience a range of abiotic edge effects (Driscoll et al. 2013). These environmental differences suggest possible links with traits, enabling formulation of testable hypotheses. For example, these changes could make species living on or above ground more vulnerable to decline, rather than those living under-ground which could retreat to avoid predators and inclement environmental conditions (Bruton et al. 2014).
Abundance in farmland frequently varied by year, implying more complex dynamics than attributable to correlates of land-conversion alone. Viviparous reptiles declined in the 2nd year on farms but rebounded in the 3rd year. In our parallel study on small mammals, house mice (Mus musculus) were 30% less abundant in parks than on farms and had very low abundance in 2011 (unpublished data). At the same time, the native predator Ningaui yvonneae was most abundant in 2011. Both species can kill reptiles. Temporal changes in the predator fauna, responding to changing resources with post-drought rainfall, could explain declines. Viviparous reptiles could be more vulnerable to the altered predation risks in 2011 than oviparous reptiles because a lower proportion live underground, and eggs of oviparous species may not be as vulnerable to predation by the relevant predator.
Only large species appeared to do better in the farmland. In some respects this is surprising; large species may be at higher risk of extinction due to smaller population sizes (Cardillo and Bromham 2001; Bohm et al. 2016) and other empirical research and meta-analyses have not found an effect of reptile body size on response to disturbance (Wang et al. 2009; Martin and Murray 2011; Doherty et al. 2020; Hu et al. 2020). However, a recent analysis of extinction-monitoring data suggests small species are more vulnerable to extinction, partly because they are prone to high variability in population growth rates (Williams et al. 2021b). Further, Palmeirim et al. (2017b) found that the lizards remaining on small, isolated islands in a hydro-electric dam in Brazil were large generalist species, which had higher dispersal and could eat a wider size-range of prey than small species. This could help explain the bias towards farmland for the large generalist agamid Pogona sp. (average abundance 10.1/site in farms, 8.5/site in parks), along with its ability to climb trees for refuge (Doherty et al. 2019). However, other large species commonly found on remnants in farmland included fossorial insectivorous snakes (Simoselaps bertholdi: 1.7/site farm, 0.3 park; Ramphotyphlops bituberculatus: 3.7 farm, 2.2 park) and a fossorial lizard-eating snake (Parasuta spectabilis: 0.7 farm, 0.4 park), all of which are nocturnal. This suggests a range of mechanisms could contribute to the success of large species, including combinations of traits that afford protection in narrow remnants in farmland such as burrowing and nocturnal activity. Further, the success of large species, including predators like snakes, may contribute to lower species richness of medium-sized species in farmland.
The response of omnivores to habitat conversion depended on region, which is likely related to fire responses of the dominant omnivore species. Of the six omnivore species, 97% of records were for two species, both of which are early to mid-successional species (Pogona sp. and Liopholis inornata, Smith et al. 2013). This explains lower abundance in the long unburnt park in the south compared with the recently burnt park in the north, although high abundance on farm remnants in the south remains unexplained. Oviparous species did not increase in 2012 on farms but did in the conservation park, a surprising result given they appeared to be more robust to fires in remnants than viviparous species. Reptile vulnerability to disturbance can depend on the timing of egg laying relative to the timing of disturbance (Pike and Stiner 2007). Possibly eggs were vulnerable to disturbances at times other than when the fire took place, or to other disturbances, such as livestock trampling.
Proximity to recently burnt or long unburnt conservation park
Low initial abundance in the north in 2010 followed by substantial increases could be explained by an interaction of fire, dispersal, and drought. The conservation park in the north was burnt in a wildfire in 2005 during the millennial drought. We suggest that slow recovery from fire during the dry years and the coincidence of our surveys with higher rainfall could account for growing numbers in the North. Our general observation of increasing abundance over the 3 years for many trait groups (Online Resource 7) likely also relates to high rainfall after the drought, similar to boom and bust patterns seen in desert mammals (Bennison et al. 2018). With evidence that the distance to conservation parks can affect some of the reptiles in our study region (this study and Williams et al. 2012), it is plausible that the combined fire and drought had spill-over effects on the adjacent remnants. We suggest that when abundance was reduced in the reserve due to fire and maintained at low levels during the drought, abundance and richness in adjacent remnants declined due to reduced immigration from the reserve. This spill-over part of the hypothesis is needed because we did not detect any significant three-way interactions of year, tenure, and region, which implies that both the remnants and reserves in the north followed similar trajectories. We emphasise this is a hypothesis for testing and acknowledge that our design does not allow us to exclude unknown and unobserved confounding factors between the north and south such as historical disturbance legacies (Gonzalez-Trujillo et al. 2021).
Combining this fire-dispersal-drought hypothesis with the acknowledgement that burnt sites were generally those in worst ecological condition enables a potential explanation for the region-year-burn interaction shown by nocturnal reptiles (Fig. 3D). It is plausible that it took the combined effects of reduced immigration from reserves due to fire and drought, and reduced patch-level success in the degraded sites to reduce abundance of nocturnal reptiles on sites selected for burning in the north.
Conclusion
Habitat loss and fragmentation are severe threats to biodiversity, and the threat escalates as intensifying land use increases the contrast between matrix and remnant (Chase et al. 2020). Our findings are consistent with this global trend; at least half of our reptile trait groups were less abundant or had fewer species in fragmented landscapes and in more isolated sites. The exceptions included trait groups with flexible life histories (omnivores and reptiles active day and night) suggesting that generalist species are most adept at surviving in isolated remnants in highly modified landscapes. Conversely, more specialised species are most vulnerable to decline (Watling and Donnelly 2007; Bohm et al. 2016).
Reptile declines globally have been associated with a broad range of traits including: small body size, large body size, habitat specialisation, ovipary, being diurnal; narrow trophic range; vertebrate prey; small geographic range; small clutch size, low population density; weak dispersal and low heat tolerance (Driscoll 2004; Watling and Donnelly 2007; Wang et al. 2009; Carvajal-Cogollo and Urbina-Cardona 2015; Bohm et al. 2016; Todd et al. 2017; Palmeirim et al. 2017a; Nowakowski et al. 2018; Doherty et al. 2020; Hu et al. 2020; Williams et al. 2021b). With this range of traits and responses, the approach we and most other studies take, correlating abundance or richness with disturbance, are revealed as useful first steps. However, these relationships are destined to remain speculative until mechanisms of vulnerability can be identified, such as by using more detailed demographic, behavioural and experimental research (e.g. Ferreira et al. 2016; Alvarez-Ruiz et al. 2021), including exploring effects of multiple correlated traits. To this end, we have summarised the hypotheses raised in our study to explain links between traits and landscape features as a guide to future research that can move beyond correlation and develop a mechanistic understanding (Table 1).
Our multifaceted survey design over multiple years suggests an equally multifaceted sequence of effects that influence reptile communities and highlights the role of non-stationary phenomena in driving spatial and temporal variation in ecological responses (Rollinson et al. 2021). We have suggested that the relatively static aspects of the landscape; habitat loss, fragmentation, and patch isolation, interact with natural fires, restoration burns and longer-term climatic trends. Reptile responses to these common landscape structures and disturbances can be mediated by traits, and detecting these relationships provide both a means of generalising the response and a testable guide to potential mechanisms.
Data availability
Data available from DRO http://dro.deakin.edu.au.
Code availability
Not relevant.
References
Alstad AO, Damschen EI (2016) Fire may mediate effects of landscape connectivity on plant community richness in prairie remnants. Ecography 39(1):36–42
Alvarez-Ruiz L, Belliure J, Pausas JG (2021) Fire-driven behavioral response to smoke in a Mediterranean lizard. Behav Ecol 32(4):662–667
Barton K (2020) MuMIn: multi-model inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn
Bateman PW, Fleming PA, Wolfe AK (2017) A different kind of ecological modelling: the use of clay model organisms to explore predator-prey interactions in vertebrates. J Zool 301(4):251–262
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Bell K, Driscoll DA, Patykowski J, Doherty TS (2021) Abundance, condition and size of a foundation species vary with altered soil conditions, remnant type and potential competitors. Ecosystems 24(6):1516–1530
Bennison K, Godfree R, Dickman CR (2018) Synchronous boom-bust cycles in central Australian rodents and marsupials in response to rainfall and fire. J Mammal 99(5):1137–1148
Blackburn G, Wright MJ (1989) Soils. In: Noble JC, Bradstock RA (eds) Mediterranean landscapes in Australia. CSIRO Publishing, Melbourne, pp 35–53
Bohannan BJM, Lenski RE (2000) The relative importance of competition and predation varies with productivity in a model community. Am Nat 156(4):329–340
Bohm M, Williams R, Bramhall HR et al (2016) Correlates of extinction risk in squamate reptiles: the relative importance of biology, geography, threat and range size. Glob Ecol Biogeogr 25(4):391–405
Bolker BM, Brooks ME, Clark CJ et al (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24(3):127–135
Bolker B, R Development Core Team (2020) bbmle: tools for general maximum likelihood estimation. R package version 1.0.23.1. http://CRAN.R-project.org/package=bbmle
Bruton MJ, McAlpine CA, Smith AG, Franklin CE (2014) The importance of underground shelter resources for reptiles in dryland landscapes: a woma python case study. Austral Ecol 39(7):819–829
Burnham KP, Anderson D (2002) Model selection and multimodel inference. Springer-Verlag, New York
Cardillo M, Bromham L (2001) Body size and risk of extinction in Australian mammals. Conserv Biol 15(5):1435–1440
Carvajal-Cogollo JE, Urbina-Cardona JN (2015) Ecological grouping and edge effects in tropical dry forest: reptile-microenvironment relationships. Biodivers Conserv 24(5):1109–1130
Chapple DG, Roll U, Bohm M et al (2021) Conservation status of the world’s skinks (Scincidae): taxonomic and geographic patterns in extinction risk. Biol Conserv 257:12
Chase JM, Blowes SA, Knight TM, Gerstner K, May F (2020) Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 584(7820):238–243
Chergui B, Pleguezuelos JM, Fahd S, Santos X (2020) Modelling functional response of reptiles to fire in two Mediterranean forest types. Sci Total Environ 732:139205
Davies RJP, Whalen MA, Mackay DA, Taylor D, Pisanu P (2013) Does soil seed bank diversity limit post-fire regeneration in small, fragmented, long-unburnt remnants of fire adapted vegetation? Biol Conserv 158:287–295
Doherty TS, Fist CN, Driscoll DA (2019) Animal movement varies with resource availability, landscape configuration and body size: a conceptual model and empirical example. Landsc Ecol. https://doi.org/10.1007/s10980-019-00795-x
Doherty TS, Balouch S, Bell K et al (2020) Reptile responses to anthropogenic habitat modification: a global meta-analysis. Glob Ecol Biogeogr 29(7):1265–1279
Driscoll DA (2004) Extinction and outbreaks accompany fragmentation of a reptile community. Ecol Appl 14(1):220–240
Driscoll DA (2008) The frequency of metapopulations, metacommunities and nestedness in a fragmented landscape. Oikos 117(8):297–309
Driscoll DA, Weir T (2005) Beetle responses to habitat fragmentation depend on ecological traits, remnant condition and shape. Conserv Biol 19(1):182–194
Driscoll DA, Henderson MK (2008) How many common reptile species are fire specialists? A replicated natural experiment highlights the predictive weakness of a fire succession model. Biol Conserv 141(2008):460–471
Driscoll DA, Lindenmayer BD (2012) Framework to improve the application of theory in ecology and conservation. Ecol Monogr 82(1):129–147
Driscoll DA, Smith AL, Blight S, Maindonald J (2012a) Reptile responses to fire and the risk of post-disturbance sampling bias. Biodivers Conserv 21:1607–1625
Driscoll DA, Whitehead CA, Lazzari J (2012b) Spatial dynamics of the knob-tailed gecko Nephrurus stellatus in a fragmented agricultural landscape. Landsc Ecol 27(6):829–841
Driscoll DA, Banks SC, Barton PS, Lindenmayer DB, Smith AL (2013) Conceptual domain of the matrix in fragmented landscapes. Trends Ecol Evol 28(10):605–613
Driscoll DA, Smith AL, Blight S, Sellar I (2020) Interactions among body size, trophic level and dispersal traits predict beetle detectability and occurrence responses to fire. Ecol Entomol 45(2):300–310
Driscoll DA, Armenteras D, Bennett AF et al (2021) How fire interacts with habitat loss and fragmentation. Biol Rev 96(3):976–998
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81(1):117–142
Ferreira CC, Santos X, Carretero MA (2016) Does ecophysiology mediate reptile responses to fire regimes? Evidence from Iberian lizards. PeerJ 4:21
Fox J, Weisberg S (2019) An {R} companion to applied regression, Third edn. Sage, Thousand Oaks. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion
Geary WL, Nimmo DG, Doherty TS, Ritchie EG, Tulloch AIT (2019) Threat webs: reframing the co-occurrence and interactions of threats to biodiversity. J Appl Ecol 56(8):1992–1997
Gentry DJ, Vierling KT (2007) Old burns as source habitats for Lewis’s woodpeckers breeding in the Black Hills of South Dakota. Condor 109(1):122–131
Gibson R, Bradstock R, Penman T, Keith DA, Driscoll DA (2015) Climatic, vegetation and edaphic influences on the probability of fire across Mediterranean Woodlands of south eastern Australia. J Biogeogr 42:1750–1760
Gonzalez-Trujillo JD, Saito VS, Petsch DK, Munoz I, Sabater S (2021) Historical legacies and contemporary processes shape beta diversity in Neotropical montane streams. J Biogeogr 48(1):101–117
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv. https://doi.org/10.1126/sciadv.1500052
Hansen NA, Sato CF, Michael D, Lindenmayer DB, Driscoll DA (2019) Predation risk for reptiles is highest at remnant edges in agricultural landscapes. J Appl Ecol 56(1):31–43
Hanski I (1998) Metapopulation dynamics. Nature 396(6706):41–49
Hu Y, Doherty TS, Jessop TS (2020) How influential are squamate reptile traits in explaining population responses to environmental disturbances? Wildl Res 47(3):249–259
Keinath DA, Doak DF, Hodges KE et al (2017) A global analysis of traits predicting species sensitivity to habitat fragmentation. Glob Ecol Biogeogr 26(1):115–127
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7(7):601–613
Leroux SJ (2019) On the prevalence of uninformative parameters in statistical models applying model selection in applied ecology. PLoS ONE 14(2):e0206711
Letnic M, Tischler M, Gordon C (2013) Desert small mammal responses to wildfire and predation in the aftermath of a La Nińa driven resource pulse. Austral Ecol 38(7):841–849
Lion MB, Garda AA, Santana DJ, Fonseca CR (2016) The conservation value of small fragments for Atlantic forest reptiles. Biotropica 48(2):265–275
Lucey JM, Tawatao N, Senior MJM et al (2014) Tropical forest fragments contribute to species richness in adjacent oil palm plantations. Biol Conserv 169:268–276
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Maindonald J, Braun J (2010) Data analysis and graphics using R. An example-based approach, 3rd edn. Cambridge University Press, Cambridge
Martin LJ, Murray BR (2011) A predictive framework and review of the ecological impacts of exotic plant invasions on reptiles and amphibians. Biol Rev 86(2):407–419
Matthews TJ (2021) On the biogeography of habitat islands: the importance of matrix effects, noncore species, and source-sink dynamics. Q Rev Biol 96(2):73–104
Meiri S (2018) Traits of lizards of the world: variation around a successful evolutionary design. Glob Ecol Biogeogr 27(10):1168–1172
Morin DJ, Yackulic CB, Diffendorfer JE et al (2020) Is your ad hoc model selection strategy affecting your multimodel inference? Ecosphere 11(1):e02997
Neilly H, Nordberg EJ, VanDerWal J, Schwarzkopf L (2018) Arboreality increases reptile community resistance to disturbance from livestock grazing. J Appl Ecol 55(2):786–799
Neuwald JL, Templeton AR (2013) Genetic restoration in the eastern collared lizard under prescribed woodland burning. Mol Ecol 22(14):3666–3679
Nimmo DG, Kelly LT, Spence-Bailey LM et al (2012) Predicting the century-long post-fire responses of reptiles. Glob Ecol Biogeogr 21(11):1062–1073
Nowakowski AJ, Watling JI, Thompson ME et al (2018) Thermal biology mediates responses of amphibians and reptiles to habitat modification. Ecol Lett 21(3):345–355
Palmeirim AF, Vieira MV, Peres CA (2017a) Herpetofaunal responses to anthropogenic forest habitat modification across the neotropics: insights from partitioning beta-diversity. Biodivers Conserv 26(12):2877–2891
Palmeirim AF, Vieira MV, Peres CA (2017b) Non-random lizard extinctions in land-bridge Amazonian forest islands after 28 years of isolation. Biol Conserv 214:55–65
Pike DA, Stiner JC (2007) Sea turtle species vary in their susceptibility to tropical cyclones. Oecologia 153(2):471–478
R Core Team (2021) R: a language and environment for statistical computing. Version 4.1.0. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available from http://www.R-project.org
Robinson AC, Heard LMB (1985) National parks. In: Twidale CR, Tyler MJ, Davies M (eds) Natural history of Eyre Peninsula. Royal Society of South Australia, Adelaide, pp 201–223
Rollinson CR, Finley AO, Alexander MR et al (2021) Working across space and time: nonstationarity in ecological research and application. Front Ecol Environ 19(1):66–72
Santos X, Cheylan M (2013) Taxonomic and functional response of a Mediterranean reptile assemblage to a repeated fire regime. Biol Conserv 168:90–98
Santos X, Mateos E, Bros V et al (2014) Is response to fire influenced by dietary specialization and mobility? A comparative study with multiple animal assemblages. PLoS ONE 9(2):e88224
Schutz AJ, Driscoll DA (2008) Common reptiles unaffected by connectivity or condition in a fragmented farming landscape. Austral Ecol 33:641–652
Scroggie MP, Peterson GNL, Rohr DH, Nicholson E, Heard GW (2019) Disturbance has benefits as well as costs for fragmented populations of a cryptic grassland reptile. Landsc Ecol 34(8):1949–1965
Segan DB, Murray KA, Watson JEM (2016) A global assessment of current and future biodiversity vulnerability to habitat loss-climate change interactions. Glob Ecol Conserv 5:12–21
Smith A, Bull CM, Driscoll DA (2012) Post-fire succession affects abundance and survival but not detectability in a knob-tailed gecko. Biol Conserv 145(1):139–147
Smith AL, Bull CM, Driscoll DA (2013) Successional specialization in a reptile community cautions against widespread planned burning and complete fire suppression. J Appl Ecol 50:1178–1186
Teasdale LC, Smith AL, Thomas M, Whitehead CA, Driscoll DA (2013) Detecting invertebrate responses to fire depends on sampling method and taxonomic resolution. Austral Ecol 38:874–883
Todd BD, Nowakowski AJ, Rose JP, Price SJ (2017) Species traits explaining sensitivity of snakes to human land use estimated from citizen science data. Biol Conserv 206:31–36
Val J, Travers SK, Oliver I, Koen TB, Eldridge DJ (2019) Recent grazing reduces reptile richness but historic grazing filters reptiles based on their functional traits. J Appl Ecol 56(4):833–842
Wang Y, Zhang J, Feeley KJ, Jiang P, Ding P (2009) Life-history traits associated with fragmentation vulnerability of lizards in the Thousand Island Lake. China Anim Conserv 12(4):329–337
Watling JI, Donnelly MA (2007) Multivariate correlates of extinction proneness in a naturally fragmented landscape. Divers Distrib 13(4):372–378
Williams JR, Driscoll DA, Bull CM (2012) Roadside connectivity does not increase reptile abundance or richness in a fragmented mallee landscape. Austral Ecol 37(3):383–391
Williams DR, Clark M, Buchanan GM, Ficetola GF, Rondinini C, Tilman D (2021a) Proactive conservation to prevent habitat losses to agricultural expansion. Nat Sustain 4(4):314–322
Williams NF, McRae L, Freeman R, Capdevila P, Clements CF (2021b) Scaling the extinction vortex: body size as a predictor of population dynamics close to extinction events. Ecol Evol 11(11):7069–7079
Wong DTY, Gruber B, Sarre SD, Osborne WS (2021) Agricultural modification to vegetation drives presence and abundance of a threatened fossorial legless lizard. Austral Ecol 46(3):437–448
Zhang D (2021) rsq: R-squared and related measures. R package version 2.2. https://CRAN.R-project.org/package=rsq
Acknowledgements
We thank the many volunteers who assisted with field work, particularly Kevin Mayes. We thank Joe Tilley from the South Australian Department of Environment and Natural Resources for implementing the burns. Sam Banks and Saul Cunningham provided valuable feedback on an earlier draft and Wade Blanchard provided statistical advice. Thanks to the landholders for access to properties and accommodation.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Australian Research Council LP0667796.
Author information
Authors and Affiliations
Contributions
JL: conception, field work, data preparation, contributions to manuscript preparation. CS: critical revisions of manuscript. DD: conception, analysis, re-analysis, writing, corresponding.
Corresponding author
Ethics declarations
Conflict of interest
Not relevant.
Ethical approval
Scientific permit K25737-1 and animal ethics permits 26/2009-M1 (Government of South Australia) and S.RE.09.09 (Australian National University).
Consent to participate
Not relevant.
Consent for publication
Not relevant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lazzari, J., Sato, C.F. & Driscoll, D.A. Traits influence reptile responses to fire in a fragmented agricultural landscape. Landsc Ecol 37, 2363–2382 (2022). https://doi.org/10.1007/s10980-022-01417-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01417-9