Abstract
Integration of landscape ecology and conservation physiology has been recommended as a potentially useful way to investigate consequences of human-induced changes in habitats for animal populations. A central goal of this paper was to examine if a simple physiological parameter displays any consistent patterns of spatio-temporal variation. Blood glucose concentration in birds reflects their high metabolic demands and may be influenced by a number of environmental factors. Therefore we present results concerning variation in glucose concentration in the blood of c. 14-day-old nestling blue tits (Cyanistes caeruleus) in central Poland in an 8-year period, 2005–2012, in two landscapes: an urban parkland and a deciduous forest. The most important findings of the study were: (1) mean levels of blood glucose varied markedly among years, most probably due to variable weather conditions, (2) glucose concentrations were significantly higher in the parkland study site than in the forest site, (3) heavier nestlings had lower glucose levels, and (4) high glucose levels were negatively correlated with fledging and breeding success. Thus we have confirmed that a consistent spatio-temporal pattern really exists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sudden changes in landscape spatial patterns exert strong pressure on animal populations. Because many of these changes are of anthropogenic origin, there is a need for focusing research efforts on understanding processes underpinning distributional patterns, including various interspecies relations and organism-environment interactions (Ellis et al. 2012). Integration of landscape ecology and conservation physiology has been recommended as a potentially useful way to investigate consequences of human-induced changes in habitats for animal populations (Ellis et al. 2012). Special attention has been paid to different measures of stress responses of animals as the most obvious effects of interest in the context of conservation physiology, because stress can lead to fitness consequences expressed especially in low-quality habitats (Ellis et al. 2012; Maron et al. 2012; Bańbura et al. 2013). It is known that food supplies vary among years and habitats, affecting nutritional state of adults as well as nestlings (Bańbura et al. 2007; Kaliński et al. 2009). Poor food conditions in low-quality habitats can lead to malnutrition, which can be a strong environmental stressor that releases endocrine responses affecting glucose concentration in the blood stream (Remage-Healey and Romero 2001; Cockrem 2007). Conservation-physiology approach may deliver early indicators of populations in trouble and in consequence give some clues how to guide habitat conservation efforts.
By analogy with mammalian physiology, it has been presumed that the main role of glucose circulating in blood of birds is to provide metabolic energy by cellular oxidation and to support the synthesis of glycogen and fatty acids as energy stores (Braun and Sweazea 2008). However, birds have been shown to be devoid of Glut 4, an insulin-stimulated glucose transport protein, to deposit only small amounts of glucose as glycogen in their liver and muscles and to use little glucose in muscle metabolism (Braun and Sweazea 2008; Scanes and Braun 2013). Fatty acids and even proteins have been demonstrated to be metabolized rather than glucose as sources of energy for flight and migration (Jenni and Jenni-Eiermann 1998; Gerson and Guglielmo 2011; Scanes and Braun 2013). Even so, blood glucose concentration in birds is roughly twice as high as in mammals of comparable body mass (Pollock 2002; Braun and Sweazea 2008; Polakof et al. 2011). The physiological mechanism of the maintenance of high glucose concentration in avian blood in comparison with mammalian blood seems to be more glucagon-related than insulin-related, as reflected in glucagon molar concentration being twofold higher than insulin concentration (Polakof et al. 2011; Scanes and Braun 2013). In typical conditions, glucose concentration reflects the actual level of carbohydrate ingestion and increases or decreases with the changing satiation state of an individual. Consequently, glucose levels are regulated by pancreatic hormones and are maintained within a relatively narrow range to avoid hyper- and hypoglycemia (Whittow 2000).
Birds are not completely uniform with respect to blood glucose concentration. Some variation among species and in relation to body size and typical food is apparent, analogously to variation in metabolic rates (Gonzalez and Hiraldo 1991; Whittow 2000; Polakof et al. 2011). Avian glucose levels are influenced by diet composition and feeding frequency, which is associated with different patterns of glucose utilization in species feeding on various food sources, with resulting considerable differences between meat-eaters and granivorous birds (Pollock 2002; Braun and Sweazea 2008; Polakof et al. 2011). For instance, carnivorous birds can maintain constant glucose levels during fasting much longer than granivorous birds, mainly because of continuous gluconeogenesis from amino acids (Pollock 2002). Glucose concentration is usually higher in smaller species, reaching its maximum in nectar-feeding hummingbirds (Beuchat and Chong 1998; Witteveen et al. 2014). In general, birds, with the exception of hummingbirds, which are non-passerines with very high mass-specific metabolic rates, can be divided roughly into two groups: passerines and non-passerines, with metabolic rates 65 % higher for passerines than non-passerines of equivalent size (Lasiewski and Dawson 1967; Nagy 2005). A comparative analysis conducted by McKechnie et al. (2006) revealed the role of phenotypic plasticity as a major contributor to avian metabolic interspecific variation in passerines and non-passerines. What is even more important in the context of the current study the spatial variation in habitat structure potentially leading to differences in trophic conditions and may influence the nutritional state of birds, with urban populations hypothesized to be more likely to be in weaker condition.
There are some reference values of glucose concentration available for free-living species (e.g. Fairbrother et al. 1990; Ferrer and Dobado-Berrios 1998; Casado et al. 2002; Davey et al. 2002; Artacho et al. 2007), but many results come from studies on poultry or captive individuals of wild species. Glucose concentration may differ markedly between captive and wild-living birds for many reasons, including possible differences in their diet (Ferrer and Dobado-Berrios 1998; Lill 2011). However, even in chickens of a limited age range, similar nutritional state and genetics there is considerable variation in the concentrations of glucose (Scanes 2008), which to some extent may result from methodological differences between studies (Scanes and Braun 2013).
Although data on glucose concentration are routinely collected in studies on migration and flight metabolism as part of blood chemistry profiles (i.e. Jenni-Eiermann and Jenni 1994; Jenni and Jenni-Eiermann 1998; Jenni-Eiermann et al. 2002), very little is known about ecological determinants and correlates of this variable in non-migrating wild birds in their natural habitats. Ruiz et al. (2002) found an interesting inter-habitat difference in blood glucose concentration of rufous-collared sparrows (Zonotricha capensis) between urban and rural habitats, with higher values in the former. For example, Minias and Kaczmarek (2013) found a correlation of glucose concentration with indicators of body condition in nestling great cormorants (Phalacrocorax carbo sinensis). Most published results concern only adult birds, while nestlings would be also of great interest (Fairbrother et al. 1990; Work 1996; Lill 2011). Nestlings of altricial passerines are usually fed by parents with specialized diets, often composed of carbohydrate-poor insects (O’Connor 1984). In this paper we analyze inter-habitat and year-to-year variation in blood glucose of nestling blue tits (Cyanistes caeruleus) to search for landscape-related spatial and temporal patterns. The existence of consistent patterns would add a new dimension to the knowledge of physiological consequences of differences between habitats, in particular, factors influencing blood glucose variation in birds.
Accordingly, the main aims of the study were: (a) to present year-to-year variation in blood glucose concentration of blue tit nestlings during an 8-year period, (b) to examine if there was a consistent difference in glucose concentration between distinct habitat types, and (c) to test if there exist fitness consequences of glucose-level differences between nestlings.
Materials and methods
Study area and field procedures
This study was carried out during 8 years, 2005–2012, as part of a long-term research project on the breeding biology of tits in central Poland. Two study areas, c. 10 km apart, were located in two different landscapes, an urban parkland and a deciduous forest. The study sites were separated by the most urbanized area of the city of Łódź. The forest study site (51°50′N; 19°29′E) in the NE part of Łódź is c. 120 ha area situated in the centre of mature deciduous forest, c. 1,250 ha in total, with oaks (Quercus robur and Q. petraea) as dominant tree species. Our forest study area is partially protected and the prevailing land use pattern is extensively recreational. The urban parkland site (51°45′N; 19°24′E), encompassing the botanical and zoological gardens, c. 80 ha in total, in SW Łódź, is characterized by a fragmented tree cover, a marked proportion of alien plant species, and intensive human disturbance over the entirety of the breeding season.
The study sites were supplied with standard wooden nestboxes (Lambrechts et al. 2010), 300 in the forest site and 200 in the parkland. During the breeding season, the nestboxes were visited at least once a week to record nesting species and basic breeding characteristics. When the first-hatched nestlings in a particular nest were 13–14 (occasionally 12–16) days old, all nestlings were banded with individually numbered metal rings; they were weighed to the nearest 0.1 g and their wing-lengths were measured to the nearest 1 mm. Subsequently, a random sample of 5 or 3 individuals per brood (5 during 2005–2006 and 3 in 2007–2012, respectively) were bled from the ulnar vein directly to 5 μL HemoCue cuvettes and analyzed in the field using a portable HemoCue Glucose 201+ photometer (HemoCue AB, Angelholm, Sweden) to measure whole blood glucose concentration (mg/dL) in peripheral blood. All field procedures were carried out between 9.00 A.M. and 2.00 P.M. Because of occasional technical problems, in some cases there are fewer actual records of glucose concentration than the numbers resulting from sample sizes. Only nestlings from first broods were included in the present study. During 8 years of the study 1,031 nestlings from 299 broods of blue tits were examined.
Because weather conditions during incubation and especially nestling stage may strongly affect food availability and feeding frequency, we examined if basic weather variables (temperature, rainfall, and humidity) indirectly influenced glucose levels of nestlings. Yearly means of glucose levels in both study sites were tested for correlation with the following average weather variables for the same years: mean daily temperature, mean daily minimum temperature, mean daily maximum temperature, the total rainfall, and mean humidity. The above correlations with weather variables were analyzed for two periods: (1) a 31-day period starting 15 days before the year mean hatching date and ending 15 days after the mean hatching date; (2) a 20-day period beginning on the earliest hatching day in a given year. The two periods overlapped and covered the nestling periods of virtually all analyzed broods. The local meteorological data for Łódź were obtained from TuTiempo.net climate data base (http://www.tutiempo.net/en/Climate/LODZ/124650.htm).
Statistical analyses
Repeatability of glucose concentrations within broods was calculated as intraclass correlation to test to what extent nestlings in broods tend to resemble one another (Zar 1996). Because the blood glucose concentration of nestlings from the same brood are likely not independent, the individual nestling values were treated as unit records and analyzed using mixed linear models, with brood ID being included as a random factor controlling for clustering; restricted maximum likelihood estimates were used and degrees of freedom were approximated by the Satterthwaite method (Heck et al. 2010). Effects of different factors on the glucose concentration were modeled in an ANCOVA style by fitting a model that included wing length as an age-controlling covariate and all factors of interest (Crawley 2002). Pearson’s bivariate correlation between residual glucose and residual mass of nestlings was used to evaluate fitness consequences of glucose concentration. Effects of blood glucose levels on fledging success (the number of fledglings in relation to the number of hatchlings in a brood) and breeding success (the number of fledglings in relation to clutch size) were calculated using a generalized linear model with binomial error distribution, logit link function, and Wald Chi squared test statistics (Crawley 2002). Post-hoc comparisons were performed using Fisher LSD tests. Relationships between yearly mean glucose levels for both study areas and between yearly means and weather variables were examined using Pearson’s linear correlation.
Mixed linear models and generalized linear models were calculated using IBM SPSS software (Heck et al. 2010). Pearson correlations were calculated in STATISTICA 10 (StatSoft Inc. 2011).
Results
Within-brood repeatability of glucose concentration
We found that the concentration of blood glucose of nestlings from the same brood tended to be consistently similar, with much variation occurring among broods, resulting in significant within-brood repeatability (R = 0.43 ± 0.03 (SE), F298;732 = 3.59, p < 0.001).
Spatial and temporal variation of blood glucose concentration
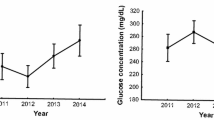
Blood glucose concentration in nestling blue tits differed between sites and years (Table 1; Fig. 1). The interaction between the site and year factors was non-significant (Table 1) and, therefore, the main factors were considered separately. The mean glucose concentration was c. 6 % higher in the parkland study area than in the forest. With respect to year-to-year variation, the highest mean concentration of glucose was recorded in 2008, slightly lower in 2009 and 2005–2007, intermediate values in 2010–2011, and the lowest level in 2012 (Fig. 1). The Fisher LSD test showed that significant differences occurred between 2007 and 2012 and between 2008 and all the other years except 2005 and 2010. Temporal changes in mean blood glucose concentration were correlated between study sites (r = 0.73, N = 8, p = 0.038).
Annual variation in mean glucose concentration of blue tit nestlings in two habitats. Means ± standard errors corrected for wing length as covariate are shown. Sample sizes of individual nestlings sampled and numbers of broods in parentheses in the parkland (top) and in the forest (bottom) are given
Impact of weather condition on mean glucose levels
For the 31-day-long period with the mean hatching date in the middle, mean daily minimum temperatures significantly negatively affected mean glucose levels in the urban parkland area (r = −0.71, N = 8, p = 0.046). Correlations between daily mean and daily mean maximum temperatures, mean humidity, total rainfall, and glucose levels for that period in the parkland were all non-significant. A similar pattern was found in the forest, but mean minimum temperatures non-significantly negatively affected yearly mean glucose levels (r = −0.69, N = 8, p = 0.057). Remaining correlations were non-significant. For the second period considered, a 20-day-long period starting with the earliest hatching date in a given year, mean daily minimum temperatures were significantly negatively correlated with yearly mean glucose levels in the forest area (r = −0.78, N = 8, p = 0.022). The analogous correlation in the parkland was non-significant (r = −0.53, N = 8, p = 0.181). Correlations between daily mean and daily mean maximum temperatures, mean humidity, sum of precipitation, and glucose levels were non-significant for either study site.
Consequences of blood glucose concentration for nestling performance
Residuals from the regression of glucose concentration on wing length were negatively correlated with residuals from the regression of body mass on wing length in nestling blue tits (r = −0.15; p = 0.008; N = 295). Fledging and breeding successes of blue tits were significantly negatively correlated with the per-brood average glucose concentration in nestling blood (Table 2).
Discussion
We found some consistent patterns of variation in the concentration of glucose in the blood of nestling blue tits. Firstly, nestlings clustered within particular broods were similar to one another in glucose concentrations, as shown by significant repeatability. Secondly, mean glucose concentrations in nestling blood differed between sites and years, with the value for the urban parkland site being regularly higher than the value for the forest site.
The range of values of mean blood glucose concentration is wide for phylogenetically diverse bird species (Beuchat and Chong 1998). Because blood glucose values are associated with metabolism, though in a non-linear way, they generally tend to be higher in smaller, altricial species (Beuchat and Chong 1998; Braun and Sweazea 2008). It is clear that many factors influence blood glucose concentration in different bird groups, with diet being one of the most important determinants. For example, hummingbirds, small-bodied and consuming mainly simple sugar-rich nectar, achieve blood glucose concentrations exceeding 700 mg/dL directly after feeding (Beuchat and Chong 1998). However, the relationship between the diet and plasma biochemistry in general is complex and different bird groups are characterized by relatively high blood glucose levels, e.g. birds of prey (Dobado-Berrios et al. 1998). The diet of nestling tits consists mainly of caterpillars, which are relatively poor in carbohydrates, but still the high concentrations of blood glucose, exceeding in some cases 400 mg/dL, were recorded for nestling blue tits in our study. Such high values in 13–14-day-old nestlings may reflect high energetic demands at this stage, when development of metabolic performance proceeds rapidly (Weathers and Siegel 1995; Bicudo et al. 2010). In fact, we know almost nothing about changes in blood biochemistry with the course of chick development in tits, but the oldest nestlings sampled in this study (c. 16-day-old) had the highest glucose levels, which is shown by a highly significant effect of the wing-length covariate in mixed models. Lill (2011) found a continuous, linear increase in nestling glucose concentration in welcome swallows (Hirundo neoxena) and spotted doves (Streptopelia chinensis). Similar results were found for protein-eating birds of prey, i.e. black vultures (Aegypius monachus) (Villegas et al. 2002) and Spanish imperial eagles (Aquila adalberti) (Ferrer and Dobado-Berrios 1998). Other studies show, however, that such an increase is not universal. For instance, in starling (Sturnus vulgaris) nestlings plasma glucose increases rapidly during the initial stage of intensive development and then reaches a plateau at a level lower than for adult individuals (Juráni et al. 2004).
Year-to-year and between-site variation found in this study could result from differences in the amount and quality of food, perhaps the occurrence of fluctuations in leaf-eating caterpillars in both study areas across years. Therefore, differences in trophic conditions can create different levels of environmental stress. In fact, year-to-year variation in caterpillar abundance was observed in our study system (Marciniak et al. 2007; Bańbura et al. 2008, 2013; Kaliński et al. 2011), as well as in other populations (Perrins 1979, Perrins 1991). However, an association between nestling glucose concentration and the trophic conditions in the environment is certainly not simple. For instance, the years 2010–2011 were richest in caterpillars during the 8-year study period, whereas 2007 was in turn the poorest one. Yet in these seasons, nestlings had medium glucose levels. Inter-annual differences in mean nestling glucose concentration imply that environmental variation among years must be considerable and, because this variation is parallel between two habitats, it can be inferred that there is some common cause. The most obvious candidate that could contribute to the year-to-year variation in nestling physiology is weather, which is greatly variable among years. Our results suggest that in springs with lower temperatures, mean glucose concentrations are higher. Effects of temperature per se is too complex to interpret and may influence many processes also in an indirect way. However, lower ambient temperatures can reduce female capacity for warming chicks efficiently and this in turn can lead to higher energy consumption by nestlings. It may also lead to lower energy consumption by nestlings since the lower ambient temperature reduces food abundance. It is possible that such conditions can act as environmental stressor and can potentially elevate glucose levels in insufficiently warmed or nourished chicks. On the other hand, an explanation for the spatial variation in glucose concentration we found seems to be more clear. There is a striking difference in glucose concentrations between the study areas. Our previous papers emphasized differences between the two sites with regard to trophic conditions, with more than twice higher caterpillar abundance in the forest being regularly recorded (Bańbura et al. 2007, 2013; Marciniak et al. 2007). Thus, the parkland as a lower-quality area creates less energetically favorable rearing condition for adults and, therefore, for nestlings. Such conditions in low-quality area could exert negative influence on different traits and generate chronic environmental stress. To test for inter-site differences in stress we analyzed variation in the heterophil-to-lymphocyte ratio (H/L) (Bańbura et al. 2013). The H/L ratio is considered as a reliable stress index, frequently used in ecological studies of birds (Ots et al. 1998; Moreno et al. 2002). We found that the H/L ratio in nestling blue tits was higher in the parkland than in the forest (Bańbura et al. 2013), which fits well to results of the present study. It is known that stressors activate secretion of corticosterone which is stimulated by hypothalamo-pituitary-adrenal (HPA) axis (Cockrem 2007). Corticosterone responses in birds are triggered by stimuli from the environment and appear 1 or 2 min after initial exposure to stressor (Cockrem 2007). Among other properties, corticosterone has the ability to increase circulating glucose concentrations, though its activity interacts with other factors (Sapolsky et al. 2000; Remage-Healey and Romero 2001; Landys et al. 2006). Corbel et al. (2010) reported stress-induced, progressive 25 % increase in plasma glucose in three different stages of development of king penguin chicks (Aptenodytes patagonicus). Similar effects were shown by Remage-Healey and Romero (2001) in adult European starlings, though at night only, which suggests that corticosterone plays a role in adjusting physiological changes to meet varying energetic demands of birds.
It should be pointed out that if the stress-induced interpretation of variation in blood glucose concentrations is correct, the trophic base and thermal conditions are likely not to be the only environmental stressors in our study system. Some other environmental factors are likely to disturb the patterns related to food base and weather conditions. The most important characteristic of our urban parkland site is an enormously high level of human activity, as the area plays a role of the main recreation area for citizens of Łódź. Crowds of visitors walk all over the area during the breeding season, which may disturb the feeding activity of breeding birds and thus the rate and regularity of feeding. Another environmental factor contributing to the difference between the parkland and forest study sites is spatial structure and species composition of vegetation. The parkland area is characterized by a patchy, strongly fragmented tree cover, with a marked proportion of exotic species and a high density of pathways, while the forest offers spatially continuous tree cover, mainly oaks of all age classes and considerable quantities of dead wood. Habitat fragmentation is known to impose difficulties on foraging birds and, therefore, to elevate stress in adult birds and, indirectly, in nestlings (Suorsa et al. 2004; Ellis et al. 2012). Hinsley et al. (2008) showed that park tits had a higher daily energy expenditure than wood tits. Obviously, testing these different effect experimentally is still needed.
Variation in nestling physiological state reflected by blood glucose concentration would be expected to have consequences for nestling fitness. Our data show that the blood glucose level is negatively correlated with nestling residual body mass. Body weight is a reliable indicator of condition in vertebrates and, particularly in birds, heavier nestlings are on average in better physiological condition (Schulte-Hostedde et al. 2005). Therefore, the negative relation between glucose level and body mass we found suggests that glucose concentration could be a kind of reverse measure of physiological condition in altricial birds, with higher than average values for nestlings in poorer physiological state. These findings are in tune with our earlier results on haemoglobin concentration as a condition index, where we demonstrated that there existed a regular pattern of blood haemoglobin concentration being on average higher in nestlings from the trophically rich forest area than in those from the urban parkland site (Bańbura et al. 2007; Kaliński et al. 2009, 2012).
In the present study, we also found that blood glucose concentrations were significant negative predictors of fledging success in blue tit broods. It supports the main idea of this paper that blood glucose concentrations exceeding a narrow average range reflect environmental stress.
Conclusions
In conclusion, we emphasize that spatial and temporal variation in blood glucose concentration is reasonably patterned in nestling blue tits, suggesting that in some circumstances it may be treated as a supplementary indicator of environmental stress. It is evident that causal connections among various environmental factors, physiological traits like glucose concentrations, and breeding success in the wild are complicated and require further investigation.
References
Artacho P, Soto-Gamboa M, Verduggo C, Nespolo R (2007) Using haematological parameters to infer the health and nutritional status of an endangered Black-necked Swan population. Comp Biochem Physiol A 147:1060–1066
Bańbura J, Bańbura M, Kaliński A, Skwarska J, Słomczyński R, Wawrzyniak J, Zieliński P (2007) Habitat and year-to-year variation in haemoglobin concentration in nestling blue tits Cyanistes caeruleus. Comp Biochem Physiol A 148:572–577
Bańbura J, Skwarska J, Kaliński A, Wawrzyniak J, Słomczyński R, Bańbura M, Zieliński P (2008) Effect of brood size manipulation on physiological condition of nestling blue tits Cyanistes caeruleus. Acta Ornithol 43:129–138
Bańbura J, Skwarska J, Bańbura M, Glądalski M, Hołysz M, Kaliński A, Markowski M, Wawrzyniak J, Zieliński P (2013) Spatial and temporal variation in heterophil-to-lymphocyte ratios of nestling passerine birds: comparison of blue tits and great tits. PLoS One 8(9):e74226
Beuchat CA, Chong C (1998) Hyperglycemia in hummingbirds and its consequences for hemoglobin glycation. Comp Biochem Physiol A 120:409–416
Bicudo JEPW, Buttemer WA, Chappel MA, Pearson JT, Bech C (2010) Ecological and environmental physiology of birds. Oxford University Press, Oxford
Braun EJ, Sweazea K (2008) Glucose regulation in birds. Comp Biochem Physiol B 151:1–9
Casado E, Balbontin J, Ferrer M (2002) Plasma chemistry in booted eagle (Hieraetus pennatus) during breeding season. Comp Biochem Physiol A 131:233–241
Cockrem JF (2007) Stress, corticosterone responses and avian personalities. J Ornithol 148(Suppl 2):S169–S178
Corbel H, Geiger S, Groscolas R (2010) Preparing to fledge: the adrenocortical and metabolic responses to stress in king penguin chicks. Funct Ecol 24:82–92
Crawley MJ (2002) Statistical computing: an introduction to data analysis using S-Plus. Wiley, Chichester
Davey C, Lill A, Baldwin J (2002) Blood glucose concentrations during breeding in short-tailed shearwaters. Emu 102:147–150
Dobado-Berrios PM, Tella JJ, Ceballos O, Donazar J (1998) Effects of age and captivity on plasma chemistry values of the Egyptian Vulture. Condor 100:719–725
Ellis R, McWhorter TJ, Maron M (2012) Integrating landscape ecology and conservation physiology. Landscape Ecol 27:1–12
Fairbrother A, Craig MA, Walker K, O’Loughlin D (1990) Changes in mallard (Anas platyrhynchos) serum chemistry due to age, sex, and reproductive condition. J Wild Dis 26:67–77
Ferrer M, Dobado-Berrios P (1998) Factors affecting plasma chemistry values of the Spanish Imperial Eagle Aquila adalberti. Comp Biochem Physiol A 120:209–217
Gerson AR, Guglielmo CG (2011) Flight at low ambient humidity increases protein catabolism in migratory birds. Science 333:1434–1436
Gonzalez JL, Hiraldo F (1991) Some hematological data from marsh harriers (Circus aeruginosus) in central Spain. Comp Biochem Physiol A 100:735–737
Heck RH, Thomas SL, Tabata LN (2010) Multilevel and longitudinal modeling with IBM SPSS. Routledge, New York
Hinsley SA, Hill RA, Bellamy PE, Harrison NE, Speakman JR, Wilson AK, Ferns PN (2008) Effects of structural and functional habitat gaps on breeding woodland birds: working harder for less. Landscape Ecol 23:615–626
Jenni L, Jenni-Eiermann S (1998) Fuel supply and metabolic constraints in migrating birds. J Avian Biol 29:521–528
Jenni-Eiermann S, Jenni L (1994) Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the garden warbler. Auk 111:888–899
Jenni-Eiermann S, Jenni L, Kvist A, Lindström Å, Piersma T (2002) Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long-distance shorebird. J Exp Biol 205:2453–2460
Juráni M, Výboh P, Zeman M, Lamošowa D, Koštál L, Blažiček P (2004) Post-hatching dynamics of plasma biochemistry in free-living European starlings (Sturnus vulgaris). Comp Biochem Physiol B 138:89–95
Kaliński A, Wawrzyniak J, Bańbura M, Skwarska J, Zieliński P, Bańbura J (2009) Haemoglobin concentration and body condition of nestlings great tits Parus major: a comparison of first and second broods in two contrasting seasons. Ibis 151:667–676
Kaliński A, Markowski M, Bańbura M, Mikus W, Skwarska J, Wawrzyniak J, Glądalski M, Zieliński P, Bańbura J (2011) Weak correlation between haemoglobin concentration and haematocrit of nestlings great tits Parus major and blue tits P. caeruleus. Ornis Fenn 88:234–240
Kaliński A, Bańbura M, Skwarska J, Wawrzyniak J, Zieliński P, Glądalski M, Markowski M, Bańbura J (2012) Parallel variation in haemoglobin concentration in nestling-rearing blue tits Cyanistes caeruleus and great tits Parus major. Acta Ornithol 47:129–136
Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, Barba E, Bouvier J-Ch, Camprodon J, Cooper CB, Dawson RD, Eens M, Eeva T, Faivre B, Garamszegi LZ, Goodenough AE, Gosler AG, Grégoire A, Griffith CG, Gustafsson L, Johnson LL, Kania W, Keišs O, Llambias PE, Mainwaring MC, Mänd R, Massa B, Mazgajski TD, Møller AP, Moreno J, Naef-Daenzer B, Nilsson J-Å, Norte AC, Orell M, Otter KA, Park CR, Perrins CR, Pinowski J, Porkert J, Potti J, Remes V, Richner H, Rytkönen S, Shiao M-T, Silverin B, Slagsvold T, Smith HG, Sorace A, Stenning MJ, Stewart I, Thompson CF, Tryjanowski P, Török J, van Noordwijk AJ, Winkler DW, Ziane N (2010) The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol 45:1–26
Landys MM, Ramenofsky M, Wingfield JC (2006) Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148:132–149
Lasiewski RC, Dawson WR (1967) A re-examination of the relation between standard metabolic rate and body weight in birds. Condor 69:13–23
Lill A (2011) Sources of variation in blood glucose concentrations of free-living birds. Avian Biol Res 4:78–86
Marciniak B, Nadolski J, Nowakowska M, Loga B, Bańbura J (2007) Habitat and annual variation in arthropod abundance affects blue tit Cyanistes caeruleus reproduction. Acta Ornithol 42:53–62
Maron M, Goulding W, Ellis RD, Mohd-Taib F-S (2012) Distribution and individual condition reveal a hierarchy of habitat suitability for area-sensitive passerine. Biodivers Conserv 21:250–2523
McKechnie AE, Freckleton RP, Jetz W (2006) Phenotypic plasticity in the scaling of avian basal metabolic rate. Proc R Soc B 273:931–937
Minias P, Kaczmarek K (2013) Concentrations of plasma metabolites as predictors of nestling condition in the Great Cormorant Phalacrocorax carbo sinensis. Ornis Fenn 90:142–150
Moreno J, Merino S, Martinez J, Sanz JJ, Arriero E (2002) Heterophil/lymphocyte ratios and heat-shock protein levels are related to growth in nestling birds. Ecoscience 9:434–439
Nagy KA (2005) Field metabolic rate and body size. J Exp Biol 208:1621–1625
O’Connor RJ (1984) The growth and development of birds. Wiley, London
Ots I, Muramagi A, Hõrak P (1998) Haematological health state indices of reproducing great tits: methodology and sources of natural variation. Funct Ecol 12:700–707
Perrins CM (1979) British tits. Collins, London
Perrins CM (1991) Tits and their caterpillar food supply. Ibis 133:49–54
Polakof S, Mommsen TP, Soengas JL (2011) Glucosensing and glucose homeostasis: from fish to mammals. Comp Biochem Physiol B 160:123–149
Pollock C (2002) Carbohydrate regulation in avian species. Semin Avian Exot Pet Med 11:57–64
Remage-Healey L, Romero ML (2001) Corticosterone and insulin interact to regulate glucose and triglyceride levels during stress in a bird. Am J Physiol Regul Integr Comp Physiol 281:R994–R1003
Ruiz G, Rosenmann M, Novoa FF, Sabat P (2002) Hematological parameters and stress index in rufous-collared sparrows dwelling in urban environments. Condor 104:162–166
Sapolsky RM, Romero ML, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppresive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Scanes CG (2008) Perspectives on analytical techniques and standarization. Poult Sci 87:2175–2177
Scanes CG, Braun E (2013) Avian metabolism: its control and evolution. Front Biol 8:134–159
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86:155–163
StatSoft Inc (2011) STATISTICA (data analysis software system), version 10. http://www.statsoft.com
Suorsa P, Helle H, Koivunen V, Huhta E, Nikula A, Hakkarainen H (2004) Effect of forest patch size on physiological stress and immunocompetence in an area-sensitive passerine, the Eurasian treecreeper (Certhia familiaris): an experiment. Proc R Soc B 271:435–440
Villegas A, Sanchez JM, Costillo E, Corbacho C (2002) Blood chemistry and haematocrit of the black vulture (Aegypius monachus). Comp Biochem Physiol A 132:489–497
Weathers WW, Siegel RB (1995) Body size establishes the scaling of avian postnatal metabolic rate: an interspecific analysis using phyloganetically independent contrast. Ibis 137:532–542
Whittow GC (ed) (2000) Sturkie’s avian physiology. Academic Press, San Diego
Witteveen M, Brown M, Downs CT (2014) Does sugar content matter? Blood plasma glucose levels in an ocassional and a specialist avian nectarivore. Comp Biochem Physiol A 167:40–44
Work TM (1996) Weights, hematology, and serum chemistry of seven species of free-ranging tropical pelagic seabirds. J Wild Dis 32:643–657
Zar JH (1996) Biostatistical analysis Prentice Hall, Upper Saddle River
Acknowledgments
All procedures were approved by the Local Ethical Committee and the State Office for Environment Protection. We thank A. Jaksa, D. Mańkowska, M. Janiszewska and J. Białek for their help and consent to conducting research in the areas under their administration. The study was founded by a grant from the Polish Ministry of Science and Higher Education No. NN304 045136 and University of Łodź (No. 506/829). We thank P. Procter for linguistic advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kaliński, A., Bańbura, M., Glądalski, M. et al. Landscape patterns of variation in blood glucose concentration of nestling blue tits (Cyanistes caeruleus). Landscape Ecol 29, 1521–1530 (2014). https://doi.org/10.1007/s10980-014-0071-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-014-0071-6