Abstract
Neuromuscular junctions are the synapses between motor neurons and skeletal muscle fibers, which mediate voluntary muscle movement. Since neuromuscular junctions are also tightly associated with the capping function of terminal Schwann cells, these synapses have been classically regarded as tripartite chemical synapses. Although evidences from sympathetic innervation of neuromuscular junctions was described approximately a century ago, the essential presence and functional relevance of sympathetic contribution to the maintenance and modulation of neuromuscular junctions was demonstrated only recently. These findings shed light on the pathophysiology of different clinical conditions and can optimize surgical and clinical treatment modalities for skeletal muscle disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Enigmatic links between sympathetic nervous system and skeletal muscle
Since the first days of modern research on skeletal muscle and neural physiology, the neuromuscular junction (NMJ) has been of prime interest and served as a model system for several fundamental principles of neuroscience, including the measurement of axonal conduction velocity (von Helmholtz 1852) and the establishment of the concepts of synapse (Sherrington 1906), quantal hypothesis (Katz 1971), synaptic vesicle recycling (Heuser and Reese 1973), and single channel recordings using patch-clamp technique (Neher et al. 1978). In general, the NMJ has often been considered as the classical example of a tripartite synapse, composed of a motor neuronal presynapse, a skeletal muscle postsynapse, and a capping by terminal Schwann cells (Sanes and Lichtman 2001; Engel and Franzini-Armstrong 2004). However, stimulatory and synchronizing effects of catecholamines, epinephrine and norepinephrine, on muscle contraction and quantal release (Kuba 1970; Khuzakhmetova and Bukharaeva 2021; Bukharaeva et al. 2021) suggested a function of sympathetic innervation in skeletal muscle and potentially at the NMJ. Further, several different autonomic disorders, such as congenital insensitivity to pain (Shorer et al. 2013), adrenal insufficiency (Martin-Grace et al. 2020), complex regional pain syndrome, and Lambert-Eaton myasthenic syndrome, share traits of muscle weakness. Finally, a correlation was observed between positive or negative pharmacological tuning of adrenoceptors and patients’ symptoms in NMJ-specific disorders, such as myasthenia gravis (Cao et al. 2020; Trillenberg et al. 2021) and some congenital myasthenic syndromes (Liewluck et al. 2011; Burke et al. 2013; Lorenzoni et al. 2013). Together, these data suggested an involvement of the sympathetic nervous system in the physiology of skeletal muscle and the NMJ.
Classical functions of the sympathetic nervous system
The Sympathetic Nervous System (SNS) of vertebrates contributes to the maintenance of homeostasis through the release of catecholamines, which modulate a variety of physiological processes and behaviors. While epinephrine is secreted into the bloodstream by the adrenal glands and acts systemically as a hormone, norepinephrine is the principal neurotransmitter released by sympathetic neurons that innervate peripheral organs and tissues throughout the body. The contacts between sympathetic neurons and their targets are established during embryonic and postnatal development (Scott-Solomon et al. 2021) and are essential for controlling diverse physiological processes, including cardiac output, blood pressure, body temperature, glycemia and immune function under basal and stress conditions. Indeed, stressful stimuli activate the SNS which maintains the animal in an excited and alerted state (Goldstein 2010) and induces a metabolic and behavioral adaptation, leading to enhanced energy supply and increased muscle performance. This so-called “fight or flight” response is also observed in invertebrates. For instance, under food deprivation conditions, Drosophila larvae exhibit increased locomotor speed and synaptic bouton numbers at NMJs. Octopamine, the invertebrate counterpart of norepinephrine, plays critical roles in these processes, but the underlying mechanisms are still poorly understood (Kamimura et al. 2019). Numerous studies, reviewed in (Navegantes et al. 2002; Roatta and Farina 2010; Berdeaux and Stewart 2012) indicate that the SNS in mammals exerts anabolic actions on skeletal muscle that are important for the preservation of tissue structure and function. The majority of the adrenergic effects on skeletal muscle mass is mediated by β2-adrenoceptors (β2-ARs) which are the predominant subtype in skeletal muscle and localized with greater density on the sarcolemma of type-I rather than type-II muscle fibers (Lynch and Ryall 2008). β2-ARs can be pharmacologically targeted by specific agonists (e.g., clenbuterol and formoterol) which are the most heavily abused substances among bodybuilders and amateur fitness athletes seeking leanness (Jessen et al. 2020). In summary, the SNS adjusts skeletal muscle physiology under acute stress and it is critical for long-term maintenance of muscle.
Morphological evidence supporting sympathetic innervation of NMJ
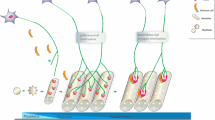
For many years, it was believed that the sympathetic innervation and adrenoceptors on skeletal muscle from mammals were restricted to vascular smooth muscle regulating muscle blood flow and blood pressure at rest and during exercise (DeLorey 2021). However, anatomical studies in the late 19th and the early 20th century using gold and silver staining of diverse muscles from different species suggested the presence of unmyelinated, thin nerve fibers and their extension into netlike structures in the immediate vicinity of NMJs (Bremer 1882; Boeke 1909, 1913; Agduhr 1920; Hines 1931). Based on their morphology, these fibers and endings were suggested to be of sympathetic origin, but clear experimental evidence for that was missing. After disputes, these findings were neglected for decades. Immunohistochemical and immunoelectron microscopic studies in the 1980ies detected tyrosine hydroxylase (TH), a marker for sympathetic neurons, in skeletal muscle fibers (Barker and Saito 1981) and more specifically, at the position of NMJs (Chan-Palay et al. 1982a, b). However, these were interpreted as signs of an extended metabolic capacity of lower motor neurons, rather than the presence of sympathetic co-innervation at NMJs. In one of these reports, muscle cross-sections were used, which showed clear, plaque-like TH immunoreactivity just next to staining of nicotinic acetylcholine receptors (nAChR) that were labeled by fluorescent α-bungarotoxin (Chan-Palay et al. 1982b). However, these samples did not allow to assign the TH immunoreactive signal to a corresponding axon. In other words, the exact nature of the TH signal remained uncertain. More than 30 years later, optical tissue clearing in combination with immunofluorescence staining and 3D confocal microscopy of diaphragm and hindlimb muscles of newborn, young and adult mice demonstrated that sympathetic neurons were indeed present in the immediate vicinity of NMJs (Khan et al. 2016; Straka et al. 2018, 2021). In diaphragms of newborn mice, TH-fluorescence signals ran alongside and within the synaptic band that is typically observed in this type of muscle (Straka et al. 2018, 2021). With increasing age, sympathetic innervation strongly ramified. Indeed, in adult diaphragm, sympathetic axons followed the larger blood vessels and then bifurcated at more or less regular intervals with one branch continuing to run along the blood vessel and another alongside the muscle fibers (Straka et al. 2021). This way, sympathetic, TH-positive axons traversed large parts of the muscle in width and height. Conversely, the axons of lower motor neurons showed a completely different pattern, i.e., these formed bundles directed exclusively to the synapse bands (Straka et al. 2021), where they branched locally to form motor units. A closer look at the distribution and morphology of TH-fluorescence signals demonstrated that sympathetic fibers apparently approach NMJs in a variety of rodent muscles, including facial muscles (Tereshenko et al. 2023), diaphragm (Khan et al. 2016; Straka et al. 2018, 2021), paw (Rodrigues et al. 2018) and hindleg muscles, like extensor digitorum longus (EDL) and tibialis anterior (Khan et al. 2016; Straka et al. 2018). Detailed analyses revealed the presence of plaque-like enrichments of TH-signals at the NMJ, which were sometimes complementary to the AChR postsynaptic labeling (Rudolf et al. 2013b; Khan et al. 2016; Straka et al. 2018). In quantitative terms, in mouse EDL muscle, TH-positive plaques at NMJs increased from roughly 40% of NMJs at birth to more than 80% in the adult (Straka et al. 2018). These data suggested that sympathetic co-innervation of NMJs is rather the rule than the exception, at least in some muscles, such as tibialis anterior and EDL. Three lines of evidence confirmed that the TH-staining in muscles was actually of sympathetic origin, i.e., retrograde tracing using fluorescent cholera toxin injected into either tibialis anterior or gastrocnemius (Rodrigues et al. 2018), anterograde tracing using DiI application at the sympathetic chain in transgenic reporter mice, and immunostaining of TH in noradrenergic neurons-reporter mice (Khan et al. 2016). In summary, these morphological data demonstrated a wide-spread distribution of sympathetic innervation in skeletal muscles in general, and of NMJs in particular.
Experimental evidence for the functional relevance of sympathetic co-innervation at NMJs
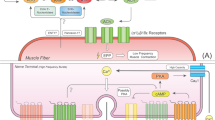
To yield evidence for a functionally active sympathetic co-innervation at NMJs, gain-of-function and loss-of-function experiments were performed in different laboratories. Gain-of-function studies used either acute electrical or optogenetic stimulation of sympathetic ganglia, or pharmacological stimulation of adrenoceptors in combination with functional readouts. Indeed, two-photon microscopic imaging in tibialis anterior muscles expressing genetically-encoded fluorescent biosensors revealed a rapid rise in postsynaptic activity of β2-ARs, elevation of local cAMP levels, and cyto-nuclear translocation of the transcriptional co-activator, PGC-1α, shortly after electrical stimulation of paravertebral sympathetic ganglia (Khan et al. 2016) (Fig. 1). On the presynaptic side, classical studies have suggested that catecholamines act at the NMJ to synchronize vesicle exocytosis and augment the release of neurotransmitter (Bowman 1981). In agreement with this notion, recent studies have demonstrated that short-term electric or optogenetic stimulation of postganglionic sympathetic neurons led to an immediate increase of the frequency of miniature-endplate potentials, but did not alter amplitude or quantal content of endplate potentials (Wang et al. 2020a, b). In contrast, long-term injection of adrenoceptor agonists, salbutamol or clenbuterol, showed not only a significant enhancement of miniature endplate potential frequency but also of endplate potential quantal content in plantar nerve-lumbricalis preparations, being these effects dependent on extracellular Ca2+ (Rodrigues et al. 2019). Furthermore, pharmacological stimulation of adrenoceptors in mouse diaphragm muscles affected spontaneous and evoked release of acetylcholine in an adrenoceptor-dependent manner: while agonists of adrenoceptors α1 and β1 decreased spontaneous release and quantal content, β2-AR stimulation increased quantal content (Tsentsevitsky et al. 2020). Finally, norepinephrine and β1-agonists were found to synchronize evoked potentials in fatigued frog cutaneous pectoris and mouse diaphragm preparations (Bukharaeva et al. 2000; Tsentsevitsky et al. 2020). At difference, norepinephrine was not active in mouse soleus muscle, but epinephrine was (Khuzakhmetova and Bukharaeva 2021). Together, these gain-of-function data suggest that sympathetic tone affects pre- and postsynaptic signaling at the mouse NMJ in an acute and long-term manner, likely in a muscle-dependent and adrenoceptor-dependent manner. Regarding the precise mechanisms, the cell types involved, and the functional roles of different adrenoceptors, there is still some debate; please refer to recent reviews (Bukharaeva et al. 2021; Delbono et al. 2021). Altogether, gain-of-function data demonstrated acute pre- and postsynaptic roles of sympathetic co-innervation at the NMJ.
Sympathetic nervous system acts upon multiple targets within skeletal muscle tissue. The schematic drawing illustrates that sympathetic innervation in skeletal muscle as a functional unit affects not only vasomotor activity, but impacts also on muscle fiber and motor neuron physiology, e.g., by controlling muscle force production and synchrony of synaptic vesicle (SV) release, respectively. At the NMJ postsynapse, sympathetic release of norepinephrine (NE) affects the protein turnover in general and that of nicotinic acetylcholine receptors (nAChR), in particular. Mechanistically, this appears to involve downregulation of proteolytic systems (atrogenes, calpain). Control of these systems might occur via adrenoceptor-mediated tuning of cAMP/PKA and/or HDAC4/myogenin axes. In addition, sympathetic innervation positively affects PGC-1alpha signaling
More long-term functions of sympathetic innervation at the NMJ were investigated by loss-of-function experiments that either involved local chemical or surgical sympathectomy. In the prior, intra-muscular injection of the neurotoxin, 6-hydroxy dopamine, over the course of two weeks led to a sustained, nearly complete deletion of sympathetic innervation of the treated muscle (Khan et al. 2016). Conversely, surgical sympathectomy at the level of the corresponding paravertebral L2/L3 sympathetic ganglia led to an almost instantaneous loss of sympathetic innervation of the entire hindleg (Silveira et al. 2014; Rodrigues et al. 2018). Both, chemical as well as surgical sympathectomy induced a similarly massive loss in muscle mass (Khan et al. 2016; Rodrigues et al. 2018), which was completely rescued by simultaneous systemic application of sympathomimetics, such as clenbuterol (Khan et al. 2016; Straka et al. 2021). Fittingly, also frequency and amplitude of miniature endplate potentials, amplitude and quantal content of endplate potentials, as well as induced muscle force were significantly reduced upon sympathectomy (Rodrigues et al. 2018). Notably, the loss in muscle force was more prominent upon stimulation of the motor nerve than upon direct stimulation of the muscles (Rodrigues et al. 2018), again suggesting an effect of sympathectomy on both, motor innervation and muscle fiber. While muscle fiber typing appeared to be unaffected upon sympathectomy, loss of cross-sectional area was observed in different fiber types, depending on which hindleg muscle was examined (Rodrigues et al. 2018). Furthermore, a careful analysis of motor neurons revealed a loss of neurofilament phosphorylation, an increase in relevant protein phosphatases types 1 and 2 A, a reduced axon diameter, and a larger variability of motor axon myelination (Rodrigues et al. 2018). Thus, loss-of-function data support a principal role of the sympathetic co-innervation for regulating NMJ-electrophysiology as well as motor neuron stability and muscle trophism.
Role of sympathetic co-innervation in protein trafficking and turnover in muscle
At the level of NMJs, sympathectomy led to massive morphological alterations at both, pre- and postsynapse, which were mainly characterized by NMJ shrinkage and reduced ramification (Khan et al. 2016; Straka et al. 2021). These signs were accompanied by an increased number of postsynaptic endocytic vesicles containing nAChR and a reduced metabolic stability of these postsynaptic receptors (Straka et al. 2021). Transcriptomic and proteomic analyses of sham-operated vs. sympathectomized hindleg muscles supported strong effects of sympathectomy on the regulation of vesicle trafficking, in particular, an enhancement of endocytosis (Rodrigues et al. 2018; Straka et al. 2021). Fittingly, mechanistic studies identified a significant reduction of the ratio of surface/total nAChR upon sympathectomy, which again argued for a loss of synaptic nAChR under this condition (Rodrigues et al. 2018). Based on previous reports, which demonstrated a key role of the E3-ubiquitin ligase, MuRF1, in endolysosomal/autophagic degradation of nAChR (Rudolf et al. 2013a; Khan et al. 2014; Carnio et al. 2014; Wild et al. 2016), the regulation of surface/total nAChR upon sympathectomy was studied in MuRF1 KO-mice. Notably, here the effect of sympathectomy on the reduction of surface/total nAChR as found in wildtype animals was blunted (Rodrigues et al. 2018). Accordingly, this was accompanied by a transient rise in MuRF1 and HDAC4 expression at 3 days after surgical sympathectomy and of myogenin (Rodrigues et al. 2018), hinting to an earlier reported gene regulatory axis involving HDAC4/5 with myogenin, miR-206, and MuRF1 as downstream targets (Moresi et al. 2010). To make the link from SNS over G-protein coupled receptor signaling to this regulatory axis, the role of Gαi2 was tested. Indeed, endogenous Gαi2 was reduced upon sympathectomy and, fittingly, overexpression of constitutively active Gαi2 in hindleg muscles blunted the rise of myogenin and the loss of surface/total nAChR upon sympathectomy (Rodrigues et al. 2018). In addition, also Gαs-mediated cAMP/PKA signaling was found to be activated by the SNS and to participate in the maintenance of the NMJ (Fig. 1). Indeed, application of norepinephrine in vivo (Silveira et al. 2020) and in vitro (Silveira et al. 2014) activated PKA and reduced MuRF1 mRNA levels in rat skeletal muscles. On top of this inhibitory effect on E3-ubiquitin ligase activity, catecholamines regulated the abundance of nAChR through calpain, a calcium-dependent protease involved in neural insults and neurodegeneration (Groshong et al. 2007). In Drosophila development, calpain modulated synaptic function through the degradation of glutamate receptors GluRIIA, but not GluRIIB, at the NMJ (Metwally et al. 2019). In C2C12 myotubes, calpain induced dispersion of AChR clusters at NMJs, an effect that was inhibited by overexpression of its endogenous inhibitor, calpastatin (Chen et al. 2007). Accordingly, in a mouse model of the slow-channel myasthenic syndrome, where Ca2+ overload occurred at the NMJ due to mutations in the genes encoding AChR subunits, transgenic overexpression of calpastatin ameliorated the symptoms and neuromuscular transmission (Groshong et al. 2007). Under physiological conditions, the gene expression of calpastatin is increased by cold exposure in rat muscles, an effect that is abrogated by plasma depletion of epinephrine (Manfredi et al. 2017). Conversely, the activity and gene expression of calpastatin is increased after β2-agonists treatment (Bardsley et al. 1992; Goncalves et al. 2012), suggesting that this molecular mechanism underlies the inhibitory effect of SNS on calpain system, which could be implicated in the maintenance of NMJ. In consonance with this notion, cAMP-inducing agents including norepinephrine (Silveira et al. 2014), cAMP-phosphodiesterase inhibitors (Baviera et al. 2007) and β2-agonists (Goncalves et al. 2012; Gonçalves et al. 2019) suppress the autophagic/lysosomal and/or calcium-dependent proteolysis (calpains), two proteolytic systems that degrade nAChR (Khan et al. 2014; Wild et al. 2016). Similarly, also the pleiotropic neuropeptide, α-calcitonin gene related peptide (CGRP), is capable of inducing cAMP production in muscle (Machado et al. 2019), and to rescue the reduction of NMJ area in denervated muscles of rats via suppression of the endolysosomal/autophagic degradation of nAChR and calpain activity (Machado et al. 2019). In summary, these data argued for a homeostatic control of synaptic nAChR levels by the sympathetic innervation through G-protein coupled receptor mediated modulation of corresponding gene expression profiles (Fig. 1).
Concluding remarks
Potential roles of sympathetic co-innervation at the NMJ in the etiology of neuromuscular disorders and in their therapy are manifold and have been discussed with respect to congenital myasthenic syndromes, myasthenia gravis, amyotrophic lateral sclerosis, spinal muscular atrophy, and sarcopenia in recent reviews (Cao et al. 2020; Rodríguez Cruz et al. 2020; Bukharaeva et al. 2021; Delbono et al. 2021; Mazzaro et al. 2023; Ohno et al. 2023). In many of these disorders, sympathomimetic drugs have been used for treatment, with variable success. Clearly, the facts that the SNS does not only innervate the NMJ but large parts of many skeletal muscles (Barker and Saito 1981; Khan et al. 2016; Straka et al. 2018, 2021; Rodrigues et al. 2018; Tereshenko et al. 2023), and that it uses (and is affected by) endocrine in parallel to neural signal transmission complicate the search for a precise understanding of causal and mechanistic relationships. Additional difficulties include the facts that (i) many adrenoceptors (and potentially further receptors, such as NPY receptors and purinergic receptors) with partially contrasting effects are involved (Bukharaeva et al. 2021) in the adrenergic effects, that (ii) the SNS is a major vasomotor control instance (Katayama and Saito 2019) that (iii) also affects skeletal muscle gene expression (Rodrigues et al. 2018; Straka et al. 2021) and (iv) acute sarcomeric force production (Sculptoreanu et al. 1993; Rudolf et al. 2006; Hotta et al. 2021). Finally, (v) sympathetic signaling molecules do not serve as bona fide neurotransmitters but as (neuro)modulators with volume transmission (Agnati et al. 1995), which renders the precise spatial attribution of norepinephrine release to its consequent cellular effects difficult. Regardless of these complications, research in the recent years has demonstrated important functions of the SNS in skeletal muscle that extend far beyond vasomotor control and future studies, for example, involving optogenetic approaches and adequate genetic mouse models, will further our insights into this fascinating field of systemic muscle research.
References
Agduhr E (1920) Sympathetic innervation of the muscles of the extremities. A histo-experimental study. Verhand D K Akad v Wetensch Amsterdam 20:1–34
Agnati LF, Zoli M, Strömberg I, Fuxe K (1995) Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69:711–726. https://doi.org/10.1016/0306-4522(95)00308-6
Bardsley RG, Allcock SM, Dawson JM et al (1992) Effect of beta-agonists on expression of calpain and calpastatin activity in skeletal muscle. Biochimie 74:267–273
Barker D, Saito M (1981) Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc R Soc Lond B Biol Sci 212:317–332
Baviera AM, Zanon NM, Carvalho Navegantes LC et al (2007) Pentoxifylline inhibits Ca2+-dependent and ATP proteasome-dependent proteolysis in skeletal muscle from acutely diabetic rats. Am J Physiol Endocrinol Metab 292:E702-8. https://doi.org/10.1152/ajpendo.00147.2006.
Berdeaux R, Stewart R (2012) cAMP signaling in skeletal muscle adaptation: hypertrophy, metabolism, and regeneration. Am J Physiol Endocrinol Metab 303:E1–17. https://doi.org/10.1152/ajpendo.00555.2011
Boeke J (1909) Ueber Eine Aus Marklosen Fasern hervorgehende Zweite Art Von Hypolemmalen Nervenendplatten bei den quergestreiften Muskelfasern Der Vertebraten. Anat Anz 35:481–484
Boeke J (1913) Die doppelte (motorische und sympathische) efferente innervation Der Quergestreiften Muskelfasern. Anat Anz 44:343–356
Bowman WC (1981) Effects of adrenergic activators and inhibitors on the skeletal muscles. In: Szekeres L (ed) Adrenergic activators and inhibitors: part II. Springer, Berlin, Heidelberg, pp 47–128
Bremer L (1882) Ueber die Endigungen Der markhaltigen und marklosen Nerven Im Quergestreiften Muskel. Archiv f mikr Anat 21:165–201
Bukharaeva ÉA, Kim KKh, Nikol’skii EE, Vyskochil F (2000) Synchronization of evoked secretion of quanta of mediator as a mechanism facilitating the action of sympathomimetics. Neurosci Behav Physiol 30:139–146. https://doi.org/10.1007/BF02463151
Bukharaeva E, Khuzakhmetova V, Dmitrieva S, Tsentsevitsky A (2021) Adrenoceptors modulate cholinergic synaptic transmission at the Neuromuscular Junction. Int J Mol Sci 22:4611. https://doi.org/10.3390/ijms22094611
Burke G, Hiscock A, Klein A et al (2013) Salbutamol benefits children with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscul Disorders: NMD 23:170–175. https://doi.org/10.1016/j.nmd.2012.11.004
Cao M, Koneczny I, Vincent A (2020) Myasthenia Gravis with antibodies against muscle specific kinase: an update on clinical features, pathophysiology and treatment. Front Mol Neurosci 13:159. https://doi.org/10.3389/fnmol.2020.00159
Carnio S, LoVerso F, Baraibar MA et al (2014) Autophagy impairment in muscle induces neuromuscular Junction Degeneration and precocious aging. Cell Rep 8:1509–1521. https://doi.org/10.1016/j.celrep.2014.07.061
Chan-Palay V, Engel AG, Palay SL, Wu JY (1982a) Synthesizing enzymes for four neuroactive substances in motor neurons and neuromuscular junctions: light and electron microscopic immunocytochemistry. Proc Natl Acad Sci U S A 79:6717–6721
Chan-Palay V, Engel AG, Wu JY, Palay SL (1982b) Coexistence in human and primate neuromuscular junctions of enzymes synthesizing acetylcholine, catecholamine, taurine, and gamma-aminobutyric acid. Proc Natl Acad Sci U S A 79:7027–7030
Chen F, Qian L, Yang Z-HH et al (2007) Rapsyn interaction with calpain stabilizes AChR clusters at the neuromuscular junction. Neuron 55:247–260. S0896-6273(07)00488-6[pii]. https://doi.org/10.1016/j.neuron.2007.06.031
Delbono O, Rodrigues ACZ, Bonilla HJ, Messi ML (2021) The emerging role of the sympathetic nervous system in skeletal muscle motor innervation and sarcopenia. Ageing Res Rev 67:101305. https://doi.org/10.1016/j.arr.2021.101305
DeLorey DS (2021) Sympathetic vasoconstriction in skeletal muscle: modulatory effects of aging, exercise training, and sex. Appl Physiol Nutr Metab 46:1437–1447. https://doi.org/10.1139/apnm-2021-0399
Engel AG, Franzini-Armstrong C (2004) Myology, 3rd edn. McGraw-Hill, New York, Chicago, San Francisco
Goldstein DS (2010) Catecholamines 101. Clin Auton Res 20:331–352. https://doi.org/10.1007/s10286-010-0065-7
Goncalves DA, Silveira WA, Lira EC et al (2012) Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt. Am J Physiol Endocrinol Metab 302:E123–E133. https://doi.org/10.1152/ajpendo.00188.2011
Gonçalves DA, Silveira WA, Manfredi LH et al (2019) Insulin/IGF1 signalling mediates the effects of β2 -adrenergic agonist on muscle proteostasis and growth. J Cachexia Sarcopenia Muscle 10:455–475. https://doi.org/10.1002/jcsm.12395
Groshong JS, Spencer MJ, Bhattacharyya BJ et al (2007) Calpain activation impairs neuromuscular transmission in a mouse model of the slow-channel myasthenic syndrome. J Clin Invest 117:2903–2912. https://doi.org/10.1172/JCI30383
Heuser JE, Reese TS (1973) Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol 57:315–344
Hines M (1931) Studies on the innervation of skeletal muscle. Am J Anat 47:1–53
Hotta H, Iimura K, Watanabe N, Shigemoto K (2021) Maintenance of contractile force of the Hind limb muscles by the somato-lumbar sympathetic reflexes. J Physiol Sci 71:15. https://doi.org/10.1186/s12576-021-00799-w
Jessen S, Solheim SA, Jacobson GA et al (2020) Beta2 -adrenergic agonist clenbuterol increases energy expenditure and fat oxidation, and induces mTOR phosphorylation in skeletal muscle of young healthy men. Drug Test Anal 12:610–618. https://doi.org/10.1002/dta.2755
Kamimura K, Odajima A, Ikegawa Y et al (2019) The HSPG Glypican regulates experience-dependent synaptic and behavioral plasticity by modulating the non-canonical BMP pathway. Cell Rep 28:3144–3156e4. https://doi.org/10.1016/j.celrep.2019.08.032
Katayama K, Saito M (2019) Muscle sympathetic nerve activity during exercise. J Physiol Sci 69:589–598. https://doi.org/10.1007/s12576-019-00669-6
Katz B (1971) Quantal mechanism of neural transmitter release. Science 173:123–126
Khan MM, Strack S, Wild F et al (2014) Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy 10:123–136. https://doi.org/10.4161/auto.26841
Khan MM, Lustrino D, Silveira WA et al (2016) Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proceedings of the National Academy of Sciences 113:746–750. https://doi.org/10.1073/pnas.1524272113
Khuzakhmetova V, Bukharaeva E (2021) Adrenaline facilitates synaptic transmission by Synchronizing Release of Acetylcholine Quanta from Motor nerve endings. Cell Mol Neurobiol 41:395–401. https://doi.org/10.1007/s10571-020-00840-3
Kuba K (1970) Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol 211:551–570. https://doi.org/10.1113/jphysiol.1970.sp009293
Liewluck T, Selcen D, Engel AG (2011) Beneficial effects of albuterol in congenital endplate acetylcholinesterase deficiency and Dok-7 myasthenia. Muscle Nerve 44:789–794. https://doi.org/10.1002/mus.22176
Lorenzoni PJ, Scola RH, Kay CSK et al (2013) Salbutamol therapy in congenital myasthenic syndrome due to DOK7 mutation. J Neurol Sci 331:155–157. https://doi.org/10.1016/j.jns.2013.05.017
Lynch GS, Ryall JG (2008) Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88:729–767. https://doi.org/10.1152/physrev.00028.2007
Machado J, Silveira WA, Gonçalves DA et al (2019) α-Calcitonin gene-related peptide inhibits autophagy and calpain systems and maintains the stability of neuromuscular junction in denervated muscles. Mol Metabolism 28:91–106. https://doi.org/10.1016/j.molmet.2019.06.024
Manfredi LH, Lustrino D, Machado J et al (2017) Adrenodemedullation activates the Ca2+-dependent proteolysis in soleus muscles from rats exposed to cold. J Appl Physiol (1985) 122:317–326. https://doi.org/10.1152/japplphysiol.00198.2016
Martin-Grace J, Dineen R, Sherlock M, Thompson CJ (2020) Adrenal insufficiency: physiology, clinical presentation and diagnostic challenges. Clin Chim Acta 505:78–91. https://doi.org/10.1016/j.cca.2020.01.029
Mazzaro A, Vita V, Ronfini M et al (2023) Sympathetic neuropathology is revealed in muscles affected by amyotrophic lateral sclerosis. Front Physiol 14:1165811. https://doi.org/10.3389/fphys.2023.1165811
Metwally E, Zhao G, Li W et al (2019) Calcium-activated calpain specifically cleaves glutamate receptor IIA but not IIB at the Drosophila Neuromuscular Junction. J Neurosci 39:2776–2791. https://doi.org/10.1523/JNEUROSCI.2213-17.2019
Moresi V, Williams AH, Meadows E et al (2010) Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 143:35–45. https://doi.org/10.1016/j.cell.2010.09.004
Navegantes LCC, Migliorini RH, do Carmo Kettelhut I (2002) Adrenergic control of protein metabolism in skeletal muscle. Curr Opin Clin Nutr Metab Care 5:281–286. https://doi.org/10.1097/00075197-200205000-00007
Neher E, Sakmann B, Steinbach JH (1978) The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch 375:219–228. https://doi.org/10.1007/BF00584247
Ohno K, Ohkawara B, Shen X-M et al (2023) Clinical and pathologic features of congenital myasthenic syndromes caused by 35 Genes-A Comprehensive Review. Int J Mol Sci 24:3730. https://doi.org/10.3390/ijms24043730
Roatta S, Farina D (2010) Sympathetic actions on the skeletal muscle. Exerc Sport Sci Rev 38:31–35. https://doi.org/10.1097/JES.0b013e3181c5cde7
Rodrigues ACZ, Messi ML, Wang Z-M et al (2018) The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta Physiol e13195. https://doi.org/10.1111/apha.13195
Rodrigues AZC, Wang Z-M, Messi ML, Delbono O (2019) Sympathomimetics regulate neuromuscular junction transmission through TRPV1, P/Q- and N-type Ca2 + channels. Mol Cell Neurosci 95:59–70. https://doi.org/10.1016/j.mcn.2019.01.007
Rodríguez Cruz PM, Cossins J, Beeson D, Vincent A (2020) The Neuromuscular Junction in Health and Disease: molecular mechanisms governing synaptic formation and homeostasis. Front Mol Neurosci 13
Rudolf R, Magalhães PJ, Pozzan T (2006) Direct in vivo monitoring of sarcoplasmic reticulum Ca2 + and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol 173:187–193. https://doi.org/10.1083/jcb.200601160
Rudolf R, Bogomolovas J, Strack S et al (2013a) Regulation of nicotinic acetylcholine receptor turnover by MuRF1 connects muscle activity to endo/lysosomal and atrophy pathways. Age (Dordrecht Netherlands) 35:1663–1674. https://doi.org/10.1007/s11357-012-9468-9
Rudolf R, Khan MM, Lustrino D et al (2013b) Alterations of cAMP-dependent signaling in dystrophic skeletal muscle. Front Physiol 4:290. https://doi.org/10.3389/fphys.2013.00290
Sanes JR, Lichtman JW (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2:791–805
Scott-Solomon E, Boehm E, Kuruvilla R (2021) The sympathetic nervous system in development and disease. Nat Rev Neurosci 22:685–702. https://doi.org/10.1038/s41583-021-00523-y
Sculptoreanu A, Scheuer T, Catterall WA (1993) Voltage-dependent potentiation of L-type Ca2 + channels due to phosphorylation by cAMP-dependent protein kinase. Nature 364:240–243
Sherrington CS (1906) The integrative action of the nervous system. Oxford Univ. Press, New Haven, London, Pxford
Shorer Z, Shaco-Levy R, Pinsk V et al (2013) Variation of muscular structure in congenital insensitivity to pain and anhidrosis. Pediatr Neurol 48:311–313. https://doi.org/10.1016/j.pediatrneurol.2012.12.015
Silveira WA, Gonçalves DA, Graça FA et al (2014) Activating cAMP/PKA signaling in skeletal muscle suppresses the ubiquitin-proteasome-dependent proteolysis: implications for sympathetic regulation. J Appl Physiol 117:11–19. https://doi.org/10.1152/japplphysiol.01055.2013
Silveira WA, Gonçalves DA, Machado J et al (2020) cAMP-dependent protein kinase inhibits FoxO activity and regulates skeletal muscle plasticity in mice. FASEB J 34:12946–12962. https://doi.org/10.1096/fj.201902102RR
Straka T, Vita V, Prokshi K et al (2018) Postnatal Development and Distribution of Sympathetic Innervation in mouse skeletal muscle. Int J Mol Sci 19:1935. https://doi.org/10.3390/ijms19071935
Straka T, Schröder C, Roos A et al (2021) Regulatory function of sympathetic innervation on the Endo/Lysosomal Trafficking of Acetylcholine Receptor. Front Physiol 12. https://doi.org/10.3389/fphys.2021.626707
Tereshenko V, Maierhofer U, Dotzauer DC et al (2023) Newly identified axon types of the facial nerve unveil supplemental neural pathways in the innervation of the face. J Adv Res 44:135–147. https://doi.org/10.1016/j.jare.2022.04.009
Trillenberg P, Katalinic A, Thern J, Graf T (2021) The risk of worsening of myasthenia by cardiovascular medication as reflected by reporting frequency. Eur J Neurol 28:2965–2970. https://doi.org/10.1111/ene.14996
Tsentsevitsky A, Nurullin L, Tyapkina O, Bukharaeva E (2020) Sympathomimetics regulate quantal acetylcholine release at neuromuscular junctions through various types of adrenoreceptors. Mol Cell Neurosci 108:103550. https://doi.org/10.1016/j.mcn.2020.103550
von Helmholtz H (1852) Messungen über Fortpflanzungsgeschwindigkeit der Reizung in den Nerven. Archiv für Anatomie, Physiologie und Wissenschaftliche Medizin 199–216
Wang Z-M, Messi ML, Grinevich V et al (2020a) Postganglionic sympathetic neurons, but not locus coeruleus optostimulation, activates neuromuscular transmission in the adult mouse in vivo. Mol Cell Neurosci Epub. https://doi.org/10.1016/j.mcn.2020.103563
Wang Z-M, Rodrigues ACZ, Messi ML, Delbono O (2020b) Aging blunts sympathetic Neuron Regulation of motoneurons synaptic vesicle release mediated by β1- and α2B-Adrenergic receptors in geriatric mice. J Gerontol Biol Sci Med Sci 75:1473–1480. https://doi.org/10.1093/gerona/glaa022
Wild F, Khan MM, Straka T, Rudolf R (2016) Progress of endocytic CHRN to autophagic degradation is regulated by RAB5-GTPase and T145 phosphorylation of SH3GLB1 at mouse neuromuscular junctions in vivo. Autophagy 12:2300–2310. https://doi.org/10.1080/15548627.2016.1234564
Acknowledgements
This work was supported by DFG (INST974/9 − 1) to R. Rudolf. This work was funded as part of the Innovation Partnership M2Aind, project DrugsData (13FH08I09IA) within the framework “Starke Fachhochschulen - Impuls für die Region (FH-Impuls). This work was supported by grants to Isis C. Kettelhut (FAPESP 2018/10089-2) and Luiz C. Navegantes (PQ CNPq: 302396/2022-5; CAPES/PROEX) who also thank João Batista Camargo Neto for assistance with the figure.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.R. had the idea for the article, performed the literature search and data analysis, and drafted and critically revised the work. I.C.K. and L.C.N. performed the literature search, drafted and critically revised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rudolf, R., Kettelhut, I.C. & Navegantes, L.C.C. Sympathetic innervation in skeletal muscle and its role at the neuromuscular junction. J Muscle Res Cell Motil 45, 79–86 (2024). https://doi.org/10.1007/s10974-024-09665-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-024-09665-9